��Ŀ����
����������ȷ����
CH3OH��6e����H2O��CO2����6H+
A����0.10 mol��L��1 NaHCO3��Һ�У�c(H+)��c(H2CO3)��c(OH��)��c(CO ) ) |
| B������ı�ȼ������890.3 kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ǣ� CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H����890.3 kJ��mol��1 |
| C����ͭ���缫���CuSO4��Һ��2Cu2+��2H2O ��� 2Cu��O2����4H+ |
| D����KOHΪ�������Һ�ļ״�ȼ�ϵ�صĸ����缫��Ӧʽ�� |
A
���������A����ȷ��B����ȼ�������ڱ���£�1mol���ʵ���������ȫȼ�������ȶ��Ļ�����ʱ���ų�������������C��ͭʧ���ӳ�ͭ���ӣ�ͭ���ӵõ��ӳ�Ϊͭ���ʣ�����Cu - 2e- =Cu������Cu2++2e-=Cu��D����������CH3OH��6e����8OH����CO2����6H2O

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
�����Ŀ
 H2(g)+I2(g),��H>0,���ң�2NO2(g)
H2(g)+I2(g),��H>0,���ң�2NO2(g)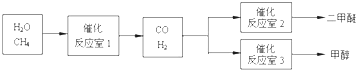
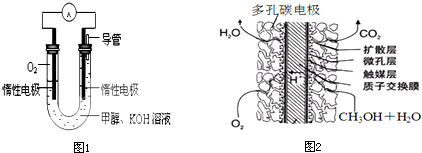
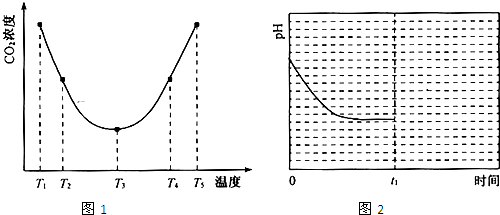
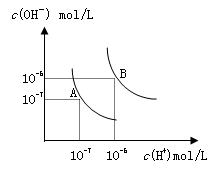
 ������˵������ȷ����
������˵������ȷ����