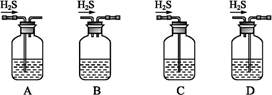
��Ŀ����
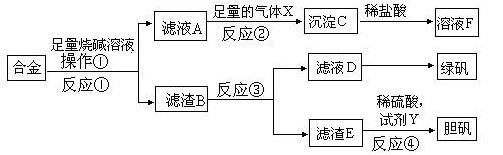
Ϊ̽����ҵ����������ͭ�Ͻ���ϵ������ã���ͬѧ��Ƶ�ʵ�鷽�����£�
��ش�
��1���̷��Ļ�ѧʽΪ ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�����ɳ��������ӷ�Ӧ����ʽ ��
��3��Ϊ�˼����ҺD�к��еĽ������ӣ������ʵ�鷽��Ϊ���Լ���ѡ���� ��
��4��������B�еμ�ϡ����ʱ�����ַ�Ӧ���ʱ�һ������۷�ӦҪ�죬��ԭ���� ��
��5����������ɫ��ѧ���գ�������E�м���ϡ������Լ�Y�Ƶ������壬�Լ�YΪ��ɫҺ�壬��Ӧ�ܵ��ܻ�ѧ����ʽΪ ������������ɫ��ѧ���գ���ѡ�Լ�YΪ1mol/L�����ᣬ��ʹ3molCuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4��������������� L��
��ʯ��

��ش�
��1���̷��Ļ�ѧʽΪ ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�����ɳ��������ӷ�Ӧ����ʽ ��
��3��Ϊ�˼����ҺD�к��еĽ������ӣ������ʵ�鷽��Ϊ���Լ���ѡ���� ��
��4��������B�еμ�ϡ����ʱ�����ַ�Ӧ���ʱ�һ������۷�ӦҪ�죬��ԭ���� ��
��5����������ɫ��ѧ���գ�������E�м���ϡ������Լ�Y�Ƶ������壬�Լ�YΪ��ɫҺ�壬��Ӧ�ܵ��ܻ�ѧ����ʽΪ ������������ɫ��ѧ���գ���ѡ�Լ�YΪ1mol/L�����ᣬ��ʹ3molCuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4��������������� L��
��ʯ��
��1��FeSO4��7H2O
��2�� 2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2 �� ��
CO2 + 2H2O + AlO2- =Al(OH)3+ HCO3-
��3�����Թ�ȡ������ҺD������Һ�еμ�KSCN��Һ�����������ٵ�����ˮ�������Ѫ��ɫ����˵����Һ�д���Fe2+��
��4��ͭ������ϡ�����γ�ԭ���
��5��Cu + H2O2 +H2SO4 = CuSO4 + 2H2O �� 2
��2�� 2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2 �� ��
CO2 + 2H2O + AlO2- =Al(OH)3+ HCO3-
��3�����Թ�ȡ������ҺD������Һ�еμ�KSCN��Һ�����������ٵ�����ˮ�������Ѫ��ɫ����˵����Һ�д���Fe2+��
��4��ͭ������ϡ�����γ�ԭ���
��5��Cu + H2O2 +H2SO4 = CuSO4 + 2H2O �� 2
��1���̷�Ϊ�����������壬��ѧʽΪ��FeSO4��7H2O
��2����Ӧ��ΪAl��NaOH��Һ�ķ�Ӧ�����ӷ���ʽΪ��
2Al+2NaOH+2H2O 2NaAlO2+3H2������Ӧ����ͨ�����������XΪCO2��CO2��H2O��AlO2-��Ӧ���ɵij���ΪAl(OH)3�����ӷ���ʽΪ��
2NaAlO2+3H2������Ӧ����ͨ�����������XΪCO2��CO2��H2O��AlO2-��Ӧ���ɵij���ΪAl(OH)3�����ӷ���ʽΪ��
AlO2-+CO2+2H2O Al(OH)3��+HCO3-(��2AlO2-+CO2+3H2O
Al(OH)3��+HCO3-(��2AlO2-+CO2+3H2O 2Al(OH)3��+CO32-)
2Al(OH)3��+CO32-)
��3����ҺD������ΪFeSO4������Fe2+��ԭ��Ϊ��Fe2+����ʹKSCN��ΪѪ��ɫ����������������Fe2+����ΪFe3+����Һ��ΪѪ��ɫ������ʵ�鷽��Ϊ�����Թ�ȡ������ҺD������Һ�еμ�KSCN����NaSCN����NH4SCN)]����Һ�����������ٵ�����ˮ����˫��ˮ����ͨ��Cl2�������Ѫ��ɫ������Һ���д���Fe2+��
��4������B�к�������ͭ�������μ�ϡ�����ͭ������ϡ�����γ���ԭ��أ�ʹ��Ӧ���ʼӿ졣
��5������EΪCu������ϡ������Լ�Y����CuSO4��������ɫ��ѧ���գ�YΪ��ɫҺ�壬���Լ�YΪH2O2����Ӧ�ܵ��ܻ�ѧ����ʽΪ��Cu+H2O2+H2SO4 CuSO4+2H2O��Cuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4����ѧ����ʽΪ��3Cu+2HNO3+3H2SO4
CuSO4+2H2O��Cuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4����ѧ����ʽΪ��3Cu+2HNO3+3H2SO4 3CuSO4++2NO��+4H2O��CuΪ3mol�������ĵ�HNO3Ϊ2mol�������������Ϊ��2mol��1mol?L?1=2L��
3CuSO4++2NO��+4H2O��CuΪ3mol�������ĵ�HNO3Ϊ2mol�������������Ϊ��2mol��1mol?L?1=2L��
��2����Ӧ��ΪAl��NaOH��Һ�ķ�Ӧ�����ӷ���ʽΪ��
2Al+2NaOH+2H2O
 2NaAlO2+3H2������Ӧ����ͨ�����������XΪCO2��CO2��H2O��AlO2-��Ӧ���ɵij���ΪAl(OH)3�����ӷ���ʽΪ��
2NaAlO2+3H2������Ӧ����ͨ�����������XΪCO2��CO2��H2O��AlO2-��Ӧ���ɵij���ΪAl(OH)3�����ӷ���ʽΪ��AlO2-+CO2+2H2O
 Al(OH)3��+HCO3-(��2AlO2-+CO2+3H2O
Al(OH)3��+HCO3-(��2AlO2-+CO2+3H2O 2Al(OH)3��+CO32-)
2Al(OH)3��+CO32-) ��3����ҺD������ΪFeSO4������Fe2+��ԭ��Ϊ��Fe2+����ʹKSCN��ΪѪ��ɫ����������������Fe2+����ΪFe3+����Һ��ΪѪ��ɫ������ʵ�鷽��Ϊ�����Թ�ȡ������ҺD������Һ�еμ�KSCN����NaSCN����NH4SCN)]����Һ�����������ٵ�����ˮ����˫��ˮ����ͨ��Cl2�������Ѫ��ɫ������Һ���д���Fe2+��
��4������B�к�������ͭ�������μ�ϡ�����ͭ������ϡ�����γ���ԭ��أ�ʹ��Ӧ���ʼӿ졣
��5������EΪCu������ϡ������Լ�Y����CuSO4��������ɫ��ѧ���գ�YΪ��ɫҺ�壬���Լ�YΪH2O2����Ӧ�ܵ��ܻ�ѧ����ʽΪ��Cu+H2O2+H2SO4
 CuSO4+2H2O��Cuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4����ѧ����ʽΪ��3Cu+2HNO3+3H2SO4
CuSO4+2H2O��Cuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4����ѧ����ʽΪ��3Cu+2HNO3+3H2SO4 3CuSO4++2NO��+4H2O��CuΪ3mol�������ĵ�HNO3Ϊ2mol�������������Ϊ��2mol��1mol?L?1=2L��
3CuSO4++2NO��+4H2O��CuΪ3mol�������ĵ�HNO3Ϊ2mol�������������Ϊ��2mol��1mol?L?1=2L��
��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ
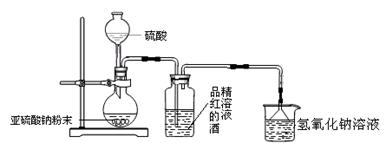

 Ca2��+
Ca2��+ KCl+2O2������д����400��Ļ�ѧ��Ӧ����ʽ ����ʾ����Ӧ��ֻ����Ԫ�صĻ��ϼ۸ı䣩��
KCl+2O2������д����400��Ļ�ѧ��Ӧ����ʽ ����ʾ����Ӧ��ֻ����Ԫ�صĻ��ϼ۸ı䣩��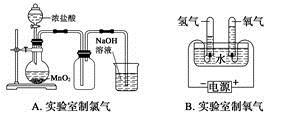

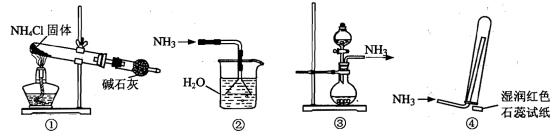

 FeSO4+H2��
FeSO4+H2��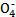 +5C2
+5C2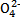 +16H+
+16H+