��Ŀ����
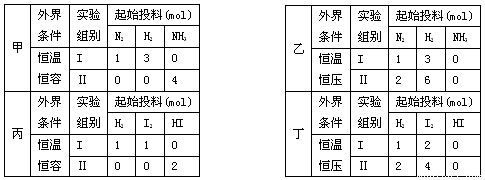
���мס�������ʵ�飬���з�ӦN2(g)+3H2(g) 2NH3(g)������������ʵ�飬���з�ӦH2(g)+I2(g)
2NH3(g)������������ʵ�飬���з�ӦH2(g)+I2(g)  2HI(g) ?H=-akJ?mol��1,ʵ����������ʼͶ�����±���ʾ�����½�����ȷ����
2HI(g) ?H=-akJ?mol��1,ʵ����������ʼͶ�����±���ʾ�����½�����ȷ����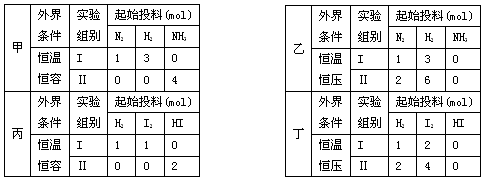
| A�������У���ƽ��ʱN2��NN3��ת���ʷֱ�Ϊ��1 �ͦ�2�����1 +��2=1 |
| B�������У�ƽ�����NH3��Ũ����ƽ���Ķ��� |
| C�������У�����ƽ��ʱ���з���Q1kJ, ��������Q2kJ����Q1+Q2=a |
| D�������У���ƽ������ʱ�䣺��С�ڢ� |
C
����

��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ
���мס�������ʵ�飬���з�ӦN2��g��+3H2��g��?2NH3��g��������������ʵ�飬���з�ӦH2��g��+I2��g��?2HI��g����H=-akJ?mol-1��ʵ����������ʼͶ�����±���ʾ�����½�����ȷ���ǣ�������
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 ��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����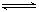 2NH3(g)������������ʵ�飬���з�ӦH2(g)+I2(g)
2NH3(g)������������ʵ�飬���з�ӦH2(g)+I2(g)