��Ŀ����
��1��������pHһ����4��9֮�䡣������Na2CO3�����ϸ�ʱ��pH���Ըߴ�10.5���������ӷ���ʽ���������ʼ��Ե�ԭ�� ������ʯ�ࣨCaSO4 2H2O������ʹ�������Խ��ͣ��йط�Ӧ�Ļ�ѧ����ʽΪ ��
2H2O������ʹ�������Խ��ͣ��йط�Ӧ�Ļ�ѧ����ʽΪ ��
��2����һ�������½������·�Ӧ��aX(g)+bY(g) cZ(g)
cZ(g)
��ͼ�Dz�ͬ�¶��·�Ӧ�ﵽƽ��ʱ����Ӧ�������Z��������� ��ѹǿ��ϵʾ��ͼ��
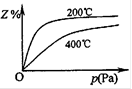
�� д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K�� �������� �ȵ����ߣ�Kֵ �����������С�����䡱��������Ӧ����ʼŨ����ͬʱ��ƽ�ⳣ��KֵԽ���� ����ĸ����ţ���
�� ����ͼ��ʾ����ͬ�¶��£��ڼס����������и�Ͷ��1molX��2molY�����������������������ij�ʼ�����Ϊ1L���ס��������ﵽƽ������ʱ�䣺�� �ң��>������<����������ͬ����ƽ��ʱX��Y��ת���ʣ��� �ҡ�
 2H2O������ʹ�������Խ��ͣ��йط�Ӧ�Ļ�ѧ����ʽΪ ��
2H2O������ʹ�������Խ��ͣ��йط�Ӧ�Ļ�ѧ����ʽΪ ����2����һ�������½������·�Ӧ��aX(g)+bY(g)
 cZ(g)
cZ(g) ��ͼ�Dz�ͬ�¶��·�Ӧ�ﵽƽ��ʱ����Ӧ�������Z��������� ��ѹǿ��ϵʾ��ͼ��

�� д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K�� �������� �ȵ����ߣ�Kֵ �����������С�����䡱��������Ӧ����ʼŨ����ͬʱ��ƽ�ⳣ��KֵԽ���� ����ĸ����ţ���
| A��X��ת����Խ�� | B����Ӧ���е�Խ��ȫ |
| C���ﵽƽ��ʱX��Ũ��Խ�� | D����ѧ��Ӧ����Խ�� |

��1��CO32��+H2O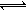 HCO3��+OH�D HCO3��+H2O
HCO3��+OH�D HCO3��+H2O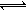 H2CO3+OH�D
H2CO3+OH�D
CaSO4 2H2O+Na2CO3��CaCO3��+Na2SO4+2H2O
2H2O+Na2CO3��CaCO3��+Na2SO4+2H2O
��2����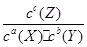 ���� AB
���� AB
�� �� ��
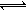 HCO3��+OH�D HCO3��+H2O
HCO3��+OH�D HCO3��+H2O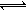 H2CO3+OH�D
H2CO3+OH�D CaSO4
 2H2O+Na2CO3��CaCO3��+Na2SO4+2H2O
2H2O+Na2CO3��CaCO3��+Na2SO4+2H2O ��2����
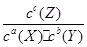 ���� AB
���� AB�� �� ��
�����������1�������ʼ��Ե�ԭ��������CO32����HCO3��ˮ�������OH�D�������ӷ���ʽΪ��
CO32��+H2O
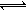 HCO3��+OH�D�� HCO3��+H2O
HCO3��+OH�D�� HCO3��+H2O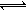 H2CO3+OH�D������ʯ�ࣨCaSO4
H2CO3+OH�D������ʯ�ࣨCaSO4 2H2O����Ca2+���Ը�CO32�������ܽ�Ⱥ�С��CaCO3���ٽ�����ƽ�������ƶ����Ӷ�ʹ�������Խ��ͣ��йط�Ӧ�Ļ�ѧ����ʽΪ��CaSO4
2H2O����Ca2+���Ը�CO32�������ܽ�Ⱥ�С��CaCO3���ٽ�����ƽ�������ƶ����Ӷ�ʹ�������Խ��ͣ��йط�Ӧ�Ļ�ѧ����ʽΪ��CaSO4 2H2O+Na2CO3��CaCO3��+Na2SO4+2H2O ����2���ٶ���aX(g)+bY(g)
2H2O+Na2CO3��CaCO3��+Na2SO4+2H2O ����2���ٶ���aX(g)+bY(g)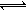 cZ(g)���仯ѧƽ�ⳣ������ʽ��K��
cZ(g)���仯ѧƽ�ⳣ������ʽ��K��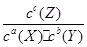 ����ͼ���Կ����÷�Ӧ�Ƿ��ȷ�Ӧ�������¶����ߣ�Kֵ���٣�����Ӧ����ʼŨ����ͬʱ��ƽ�ⳣ��KֵԽ��˵����Ӧ���е�Խ��ȫ��ͬʱX������Y��ת����Խ�ߣ���ѡAB�����ɷ�Ӧ�������Z�����������ѹǿ��ϵʾ��ͼ�����Կ�����a+b>c���������������ij�ʼ�����Ϊ1Lʱ�����ڼ��ǹ̶�������ҵ�����ɱ仯����a+b>c�������ҵķ�Ӧ���ʴﵽƽ���ʱ��̣���������ʱ�䣺�ף��ң�ͬ��������a+b>c����ת���ʣ��ҡ�
����ͼ���Կ����÷�Ӧ�Ƿ��ȷ�Ӧ�������¶����ߣ�Kֵ���٣�����Ӧ����ʼŨ����ͬʱ��ƽ�ⳣ��KֵԽ��˵����Ӧ���е�Խ��ȫ��ͬʱX������Y��ת����Խ�ߣ���ѡAB�����ɷ�Ӧ�������Z�����������ѹǿ��ϵʾ��ͼ�����Կ�����a+b>c���������������ij�ʼ�����Ϊ1Lʱ�����ڼ��ǹ̶�������ҵ�����ɱ仯����a+b>c�������ҵķ�Ӧ���ʴﵽƽ���ʱ��̣���������ʱ�䣺�ף��ң�ͬ��������a+b>c����ת���ʣ��ҡ������������⿼�黯ѧƽ����ƶ����Ǹ߿��Ŀ����ص㣬�������Ϣ���ϴ����ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
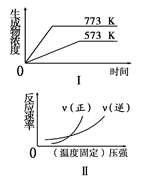
 2NH3(g)����H��0
2NH3(g)����H��0 2C��������H<0��ƽ����ϵ������˵���������
2C��������H<0��ƽ����ϵ������˵��������� 2NH3����ƽ��״̬����
2NH3����ƽ��״̬���� 2NH3(g) ��H����92.4 kJ��mol-1������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼʾ������˵���������
2NH3(g) ��H����92.4 kJ��mol-1������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼʾ������˵���������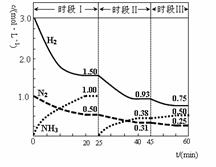
 2SO3(g) (��H< 0)
2SO3(g) (��H< 0)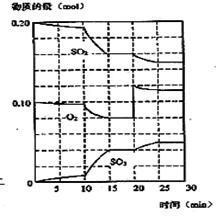
 Fe3O4(s)��4H2(g)������ʼͶ��3mol����4molˮ�������Ϊ0.5L���ܱ������з�Ӧ��ƽ��ʱ���������ʵ���Ũ��4mol��L��1,��ѧƽ�ⳣ��Ϊ
Fe3O4(s)��4H2(g)������ʼͶ��3mol����4molˮ�������Ϊ0.5L���ܱ������з�Ӧ��ƽ��ʱ���������ʵ���Ũ��4mol��L��1,��ѧƽ�ⳣ��Ϊ