��Ŀ����
��һ�����Ϊ2L���ܱ������У������·������з�Ӧ��
����Fe(s) �� CO2(g) FeO(s) �� CO(g) + Q kJ
FeO(s) �� CO(g) + Q kJ
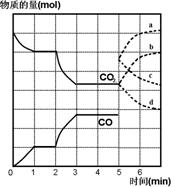
����CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ͼ��ʾ��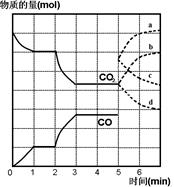
(1)����Ӧ��1minʱ��һ�δﵽƽ��״̬�����������������3.2g����CO��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʦ�(CO)=_________��
(2)����Ӧ������2minʱ����ֻ�ı�һ�����������߷����ı仯��ͼ��ʾ��3minʱ�ٴδﵽƽ�⣬��Q 0�����������������=��������һ��ƽ����ڶ���ƽ���ƽ�ⳣ����ȣ�K1 K2��(���������������=��)��
(3)��5minʱ�ٳ���һ������CO(g)��ƽ�ⷢ���ƶ�������˵����ȷ���� ����д��ţ���
a����(��)��������С b����(��)�ȼ�С������
c����(��)��������С d����(��)�ȼ�С������
��ʾn(CO2)�仯��������________����дͼ�����ߵ���ĸ��ţ���
(4)�����ù�̬���ʵ��й���������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬��
______________________________________________________________________��
0.1mol/(L��min)��2�֣�
��������1��1�𣬹�2�֣�
c �� b ��1��1�𣬹�2�֣�
Fe(��FeO)������(�����ʵ���)���ֲ��䣻�������������ֲ��䡣��2�֣�
����

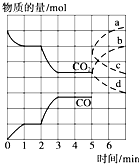 ��һ�����Ϊ2L���ܱ������У������·�����Ӧ��Fe��s��+CO2��g��?FeO��s��+CO��g��������CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ϵ��ͼ��ʾ��
��һ�����Ϊ2L���ܱ������У������·�����Ӧ��Fe��s��+CO2��g��?FeO��s��+CO��g��������CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ϵ��ͼ��ʾ�� FeO(s) �� CO(g) + Q kJ
FeO(s) �� CO(g) + Q kJ