��Ŀ����
��8�֣� X��Y��Z��W��Ԫ�����ڱ�ǰ�������е����ֳ���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
| Z | Z��Yͬ����,Z�ĵ縺�Դ���Y |
| W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��1��Yλ��Ԫ�����ڱ������������ڱ����������壬 ����
��2��Y��Z������������Ӧ��ˮ��������Խ�ǿ������������������д��ѧʽ����
��3��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��������������������������
��4��������XO��YO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����Y��
��֪��
XO(g)+![]() O2(g)=XO2(g)
O2(g)=XO2(g) ![]() H=��283.0 kJ��mol��1
H=��283.0 kJ��mol��1
Y(g)+ O2(g)=YO2(g) 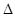 H=��296.0 kJ��mol��1
H=��296.0 kJ��mol��1
�˷�Ӧ���Ȼ�ѧ����ʽ������������������������������������������
��8�֣�
(1) 3 �� VIA ��p ��2��HClO4 ��ÿ��1�֣�
(3)[Ar]3d104s1 ��1s22s22p63s23p63d104s1 ��2�֣�
(4)2CO(g)+SO2(g)=S(s)+2CO2(g)�� ��H=��270kJ/mol��2�֣�
����:

X��Y��Z��W��Ԫ�����ڱ�ǰ�����ڵij���Ԫ�أ��������Ϣ���±�:
|
X |
X��һ�ֺ��ص�������Ϊ56��������Ϊ30 |
|
Y |
��ˮ��Ԫ�غ�����ߵĽ���Ԫ�� |
|
Z |
���³�ѹ�£�Z�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
|
W |
�۵��ӵ��Ų�ʽΪ3s23p3 |
��1��ZԪ����Ԫ�����ڱ��е�λ��Ϊ ��Z�ĵ縺�Ա�W�� �����С������
��2��XԪ���Ļ�̬ԭ�ӵ����Ų�ʽ�� _ ������___���˶�״̬��ͬ�ĵ��ӡ���Ԫ�صİ�ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ ��
��3��Y�ĵ����ڹ�ҵ����;�㷺����ҵ����YW��ȡY���ʵĻ�ѧ����ʽΪ ��
��4�����ʵ����֤Z�ͣķǽ����Ե�ǿ���� ��
��14�֣�X��Y��Z��W��Q��ԭ��������������Ķ���������Ԫ�أ������Ϣ���±���
|
Ԫ �� |
�����Ϣ |
|
X |
Xԭ�Ӻ��������������Ǵ�����2�� |
|
Y |
Y����̬�⻯���ˮ��Һ�������� |
|
Z |
Z�ǵؿ��к������Ľ���Ԫ�� |
|
W |
���³�ѹ�£�W�ĵ����ǵ���ɫ���� |
|
Q |
���� |
����������Ϣ�ش��������⣺
(1)Ԫ��Q��Ԫ�����ڱ��е�λ��______________________________��
(2)Y�������̬�⻯���ˮ��Һ����H2O2������Ӧ������ﲻ��Ⱦ��������ѧ����ʽΪ______________________________________������Ԫ�ط��ű�ʾ����ͬ��
(3)X����Ԫ����ɵĻ����������6��ԭ�ӣ���ṹʽΪ ��
(4)��֪��X(s)+O2(g) =XO2(g) ��H = -393.5��J��mol-1
2X(s)+O2(g) =2XO(g) ��H = -221.0��J��mol-1
��XO��ȼ���ȵ��Ȼ�ѧ����ʽ__________________________________________________.
(5)Ԫ��Y����Ԫ���γɵ�����������ң����Һ�Z�������ӵĻ����Һ�м������Na2O2�� ����Na2O2�����ʵ����������������ͼ��ʾ��ϵ��

д���йط�Ӧ���ӷ���ʽ��(ÿ��ֻ��һ�����ӷ���ʽ��ʾ)
o��a��
a��b�� ��

