��Ŀ����
��1���������NaCl��MgCl2��AlCl3������Һ�ֱ������������ʺ���Ũ�ȵ�AgNO3��Һǡ����ȫ��Ӧ����NaCl��MgCl2��AlCl3������Һ�����ʵ���Ũ��֮����______��
��2����������CH4��NH3��Ƚϣ����ǵķ��Ӹ�����Ϊ______�� ���ǵ���ԭ�Ӹ�����Ϊ______
��3��V mL Al2��SO4��3��Һ�У�����Al3+ag ȡV/2mL ��Һϡ�͵�4V mL����ϡ�ͺ���Һ��SO42-�����ʵ���Ũ��Ϊ______��
�⣺��1���������NaCl��MgCl2��AlCl3������Һ�ֱ������������ʺ���Ũ�ȵ�AgNO3��Һǡ����ȫ��Ӧ�������������ʵ�����ͬ��������AgCl�����ʵ�����ͬ����AgClΪ1mol�����������غ��֪��
n��NaCl��=1mol��n��MgCl2��= mol��n��AlCl3��=
mol��n��AlCl3��= mol����Һ�����ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ�
mol����Һ�����ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ�
��NaCl��MgCl2��AlCl3������Һ�����ʵ���Ũ��֮���ǣ�1mol�� mol��
mol�� mol=6��3��2���ʴ�Ϊ��6��3��2��
mol=6��3��2���ʴ�Ϊ��6��3��2��
��2��������ͬ��������Ŀ֮�ȵ���Ħ������֮�ȣ��ʵ�������CH4��NH3�ķ��Ӹ�����Ϊ17g/mol��16g/mol=17��16������Hԭ�Ӹ���֮��Ϊ17��4��16��3=17��12��
�ʴ�Ϊ��17��16��17��12��
��3��V mL Al2��SO4��3��Һ��Al3+�����ʵ���Ϊ =
=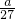 mol����V/2mL��Һ��Al3+�����ʵ���Ϊ
mol����V/2mL��Һ��Al3+�����ʵ���Ϊ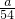 mol���ɵ���غ��֪3n��Al3+��=2n��SO42-������n��SO42-��=
mol���ɵ���غ��֪3n��Al3+��=2n��SO42-������n��SO42-��= n��Al3+��=
n��Al3+��= ��
��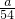 mol=
mol=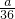 mol����ϡ�ͺ���Һ��SO42-�����ʵ���Ũ��Ϊ
mol����ϡ�ͺ���Һ��SO42-�����ʵ���Ũ��Ϊ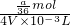 =
=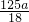 mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ��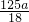 mol/L��
mol/L��
��������1���������NaCl��MgCl2��AlCl3������Һ�ֱ������������ʺ���Ũ�ȵ�AgNO3��Һǡ����ȫ��Ӧ�������������ʵ�����ͬ��������AgCl�����ʵ�����ͬ����AgClΪ1mol�������������غ����n��NaCl����n��MgCl2����n��AlCl3������Һ�����ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ�
��2������n= ��֪��������ͬ��������Ŀ֮����Ħ�������ʷ��ȣ����ݷ�Ӧ�к���Hԭ����Ŀ�����㺬��Hԭ�ӵĸ���֮�ȣ�
��֪��������ͬ��������Ŀ֮����Ħ�������ʷ��ȣ����ݷ�Ӧ�к���Hԭ����Ŀ�����㺬��Hԭ�ӵĸ���֮�ȣ�
��3������n= ����V mL Al2��SO4��3��Һ��Al3+�����ʵ�������������V/2mL��Һ��Al3+�����ʵ������ɵ���غ��֪3n��Al3+��=2n��SO42-�����ݴ˼���n��SO42-�����ٸ���c=
����V mL Al2��SO4��3��Һ��Al3+�����ʵ�������������V/2mL��Һ��Al3+�����ʵ������ɵ���غ��֪3n��Al3+��=2n��SO42-�����ݴ˼���n��SO42-�����ٸ���c= ����ϡ�ͺ�SO42-Ũ�ȣ�
����ϡ�ͺ�SO42-Ũ�ȣ�
���������⿼�����ʵ���Ũ�ȵ��йؼ��㣬�Ѷ��еȣ�ע��Թ�ʽ��������������ã�
n��NaCl��=1mol��n��MgCl2��=
 mol��n��AlCl3��=
mol��n��AlCl3��= mol����Һ�����ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ�
mol����Һ�����ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ���NaCl��MgCl2��AlCl3������Һ�����ʵ���Ũ��֮���ǣ�1mol��
 mol��
mol�� mol=6��3��2���ʴ�Ϊ��6��3��2��
mol=6��3��2���ʴ�Ϊ��6��3��2����2��������ͬ��������Ŀ֮�ȵ���Ħ������֮�ȣ��ʵ�������CH4��NH3�ķ��Ӹ�����Ϊ17g/mol��16g/mol=17��16������Hԭ�Ӹ���֮��Ϊ17��4��16��3=17��12��
�ʴ�Ϊ��17��16��17��12��
��3��V mL Al2��SO4��3��Һ��Al3+�����ʵ���Ϊ
 =
=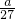 mol����V/2mL��Һ��Al3+�����ʵ���Ϊ
mol����V/2mL��Һ��Al3+�����ʵ���Ϊ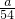 mol���ɵ���غ��֪3n��Al3+��=2n��SO42-������n��SO42-��=
mol���ɵ���غ��֪3n��Al3+��=2n��SO42-������n��SO42-��= n��Al3+��=
n��Al3+��= ��
��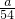 mol=
mol=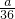 mol����ϡ�ͺ���Һ��SO42-�����ʵ���Ũ��Ϊ
mol����ϡ�ͺ���Һ��SO42-�����ʵ���Ũ��Ϊ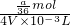 =
=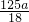 mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ��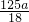 mol/L��
mol/L����������1���������NaCl��MgCl2��AlCl3������Һ�ֱ������������ʺ���Ũ�ȵ�AgNO3��Һǡ����ȫ��Ӧ�������������ʵ�����ͬ��������AgCl�����ʵ�����ͬ����AgClΪ1mol�������������غ����n��NaCl����n��MgCl2����n��AlCl3������Һ�����ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ�
��2������n=
 ��֪��������ͬ��������Ŀ֮����Ħ�������ʷ��ȣ����ݷ�Ӧ�к���Hԭ����Ŀ�����㺬��Hԭ�ӵĸ���֮�ȣ�
��֪��������ͬ��������Ŀ֮����Ħ�������ʷ��ȣ����ݷ�Ӧ�к���Hԭ����Ŀ�����㺬��Hԭ�ӵĸ���֮�ȣ���3������n=
 ����V mL Al2��SO4��3��Һ��Al3+�����ʵ�������������V/2mL��Һ��Al3+�����ʵ������ɵ���غ��֪3n��Al3+��=2n��SO42-�����ݴ˼���n��SO42-�����ٸ���c=
����V mL Al2��SO4��3��Һ��Al3+�����ʵ�������������V/2mL��Һ��Al3+�����ʵ������ɵ���غ��֪3n��Al3+��=2n��SO42-�����ݴ˼���n��SO42-�����ٸ���c= ����ϡ�ͺ�SO42-Ũ�ȣ�
����ϡ�ͺ�SO42-Ũ�ȣ����������⿼�����ʵ���Ũ�ȵ��йؼ��㣬�Ѷ��еȣ�ע��Թ�ʽ��������������ã�

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ�����˵����ȷ���ǣ�������
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ�����˵����ȷ���ǣ�������