��Ŀ����
��֪ǰ����������Ԫ��A��B��C��D��E��F��ԭ������֮��Ϊ107�������ǵĺ˵������������Bԭ�ӵ�p�������������⻯��е���ͬ��Ԫ������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A��C���γ�A2C�����ӻ�������е��������������һ�����Ӳ㣬E4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ����ش��������⣺
��1��A��B��C��D�ĵ�һ��������С�����˳����____________����Ԫ�ط��ţ�
��2��������BD3�ķ��ӿռ乹�Ϳ�����Ϊ_________��B��ԭ�ӹ���ӻ�����Ϊ________��
��3����֪FԪ���������ں���ƫ��ʱ����Ӱ��O2�����ڵ��������䡣��֪F2����KCN��Һ��Ӧ��F��CN��2���������������KCN��Һʱ�����ܽ⣬����������F�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ______��CN����___________��һ�ַ��ӣ���Ϊ�ȵ����壬��1��CN����������ĿΪ___________��
��4��EO2��̼�ᱵ������״̬�·�Ӧ�����þ���ľ����ṹ��ͼ��ʾ����÷�Ӧ�Ļ�ѧ����ʽΪ________
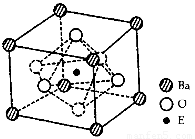
�ڸþ����У�E4��������Ϊ��Ϊ____________�����þ����߳�Ϊa nm�� ����þ�����ܶ�Ϊ__________g��cm3�������ӵ�����ΪNA��
��1��Na<S<P<Cl
��2��������? sp3
��3��? 3d64s2? N2? 2
��4��TiO2+BaCO3 BaTiO3+CO2�� ;?? 6 ;?? 233/[NA����a��10-7��3]
BaTiO3+CO2�� ;?? 6 ;?? 233/[NA����a��10-7��3]
��������
�����������1��Bԭ�ӵ�p���������������ĵ����Ų�ʽΪ1s22s22p3. BΪNԪ�ء������⻯����˵���ṹ���Ƶ����ʣ���Է�������Խ���Ӽ���������Խ�����ʵ��۷е��Խ�ߡ���HF��H2O��NH3�ķ���֮����˴��ڷ��Ӽ��������⣬������һ�ֽ�������������������˷��Ӽ������ã�ʹ���ǵ��۷е���ͬ��Ԫ���γɵ��⻯������ߣ����ַ�������NH3�ķе���ͬ��������ߵģ����������⡣������������Ų�ʽ��1s22s22p63s23p3��BԪ��ΪPԪ�ء�����Ϊ���⻯��е���ͬ��Ԫ������͵ķ������⣻���BԪ��ΪPԪ�ء�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ��������D�ĵ����Ų�ʽΪ1s22s22p63s23p5. DΪCl.Ԫ�ء�A��C���γ�A2C�����ӻ�������е��������������һ�����Ӳ㣬��A��C��Ԫ�����ڱ���λ��ͬһ���ڣ���ϻ�����ļ����ǵ�ԭ����������ClС��֪ʶ��ȷ��AΪNaԪ�أ�CΪSԪ�ء�E4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ����EΪ22��Ԫ��TiԪ�ء�A��B��C��D��E��F��ԭ������֮��Ϊ107������F��ԭ������Ϊ��107-11-15-16-17-22=26��F��FeԪ�ء���1�����ڵ��Ӳ���Խ���Ԫ�أ�ԭ�Ӻ���ĵ�����Խ�࣬ԭ�Ӱ뾶ԽС��ԭ��ʧȥ���Ӿ�Խ�ѣ��������ܾ�Խ������Na��P��S��Cl�ĵ�һ��������С�����˳����Na<S<P<Cl����2�ڻ�����PCl3�ķ����У�ÿ��Pԭ��������Clԭ���γ��������ۼ�����Pԭ���ϻ���һ�Թ¶Ե��ӡ�����PCl3�ķ��ӿռ乹�Ϳ�����Ϊ������. Pԭ�ӵ��ӻ���ʽΪsp3 ����3��Feԭ�ӵĻ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2�����̬Fe�ļ۵����Ų�ʽΪ3d64s2����������ͬ��ԭ����Ҳ��ͬ�����ͽ����ȵ����塣CN���ĵ�����Ϊ14��ԭ����Ϊ2��������CN����Ϊ�ȵ�����ķ���ΪN2���ȵ�����ṹ���ƣ�����Ҳ���ơ�������N2��2��Nԭ�ӹ������Ե��ӣ�һ����?����2��������������CN���е�����Ҳ��2������4���ɾ����Ľṹʾ��ͼ��Ͼ�̯����֪����ÿ�������к��У�Ti��1;? Ba��8��1/8=1;? O:6��1/2=3.���Ըû�����Ļ�ѧʽΪBaTiO3����TiO2��̼�ᱵ������״̬�·�Ӧ�Ļ�ѧ����ʽΪTiO2+BaCO3 BaTiO3+CO2�����ھ�����ÿ��Tiԭ����Χ��6��Oԭ������������ȶ����������6��Oԭ�ӹ��ɵ����������塣������λ��Ϊ6.������һ��������ֻ����һ��BaTiO3�����Ծ�����ܶ�
BaTiO3+CO2�����ھ�����ÿ��Tiԭ����Χ��6��Oԭ������������ȶ����������6��Oԭ�ӹ��ɵ����������塣������λ��Ϊ6.������һ��������ֻ����һ��BaTiO3�����Ծ�����ܶ�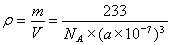 ��
��
���㣺����Ԫ�ص��ƶϡ�ԭ��ʧȥ���ӵ����ס�ԭ�ӵ��ӻ���ʽ�����ӵĽṹ�Ϳռ乹�͡�ԭ�Ӿ���Ļ�ѧʽ���Ƶ����ܶȵļ����֪ʶ��

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�


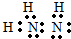
 ԭ�ӵĸþ����к��еĹ��ۼ�����ĿΪ_________��
ԭ�ӵĸþ����к��еĹ��ۼ�����ĿΪ_________�� ����û�����Ļ�ѧʽΪ_________�����п��ܺ��еĻ�ѧ������Ϊ___________����֪�û�������ˮ�п��Է���ˮ�⣬��ˮ������ӷ���ʽΪ_______________________��
����û�����Ļ�ѧʽΪ_________�����п��ܺ��еĻ�ѧ������Ϊ___________����֪�û�������ˮ�п��Է���ˮ�⣬��ˮ������ӷ���ʽΪ_______________________��