��Ŀ����
�����ճ���������;��㡢�������Ľ������ϡ�
(1)�����£�������������ʢװŨ�����ԭ���� ��
��2��ijʵ��С��������ͼװ����֤����ˮ�����ķ�Ӧ��
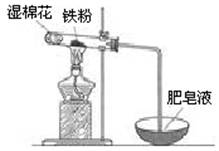
��ʪ���������� ���Թ��з�Ӧ�Ļ�ѧ����ʽ�� ��
��ʵ�������ȡ��������Ӧ��Ĺ������Թ��У�����������ᣬ������ȫ�ܽ⣬������Һ�д��ڵ��������� ������ţ���
a��һ����Fe2+��H+��Fe3+ b��һ����Fe2+��H+��������Fe3+
c��һ����Fe2+��Fe3+�������� H+ d��һ����Fe3+��H+��������Fe2+
��3������ȡһ��������������������Ũ�����У����ȣ���ַ�Ӧ���ռ����塣���ⶨ�����к���SO2��CO2��H2��
�� ����Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
�� �����л���CO2��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�� ��672 mL����״�����ռ���������ͨ��������ˮ�У�������Ӧ��
SO2 + Br2 + 2H2O = 2HBr + H2SO4��Ȼ���������BaCl2��Һ����ϴ�ӡ�����õ�����4.66 g���ɴ���֪�ռ�����������SO2����������� ��
��1��Ũ�������ǿ�����ԣ�������������һ�����ܵ�����Ĥ
��2�����ṩˮ���� 3Fe+4H2O(g)  Fe3O4+4H2
��b
Fe3O4+4H2
��b
��3����2Fe+6H2SO4(Ũ)  Fe2(SO4)3+3SO2��+6H2O
Fe2(SO4)3+3SO2��+6H2O
��C+2H2SO4(Ũ)  CO2��+2SO2��+2H2O
��66.7%
CO2��+2SO2��+2H2O
��66.7%
����������1������Ũ�������ǿ�����ԣ�����Ũ�����з����ۻ���������������һ�����ܵ�����Ĥ�����Կ�����������ʢװŨ���ᡣ
��2���ٷ�Ӧ�ﻹ��ˮ����������ʪ�����������ṩˮ��������Ӧ�ķ���ʽΪ3Fe+4H2O(g)  Fe3O4+4H2 ��
Fe3O4+4H2 ��
�����������������к��У�2�ۺͣ�3�۵�������������������ǿ�������ӵģ�����������������ʣ��ĵ������������Һ��һ�������������ӣ�����ֻ��ѡ��b��ȷ��
��3�����ڼ��ȵ������£�Ũ���������Ӧ�ķ���ʽΪ2Fe+6H2SO4(Ũ)  Fe2(SO4)3+3SO2��+6H2O��
Fe2(SO4)3+3SO2��+6H2O��
���������к�������̼��̼�ܱ�Ũ������������CO2������ʽΪC+2H2SO4(Ũ)  CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O��
�۰�ɫ���������ᱵ�����ʵ�����0.02mol�������Sԭ���غ��֪��SO2��0.02mol������������SO2�����������0.02��0.03��100����66.7����

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д� �����ճ���������;��㡢�������Ľ������ϣ�
�����ճ���������;��㡢�������Ľ������ϣ�
