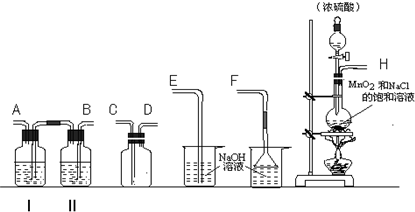
��Ŀ����
ʵ��������ʳ�κ�̼������Ʊ�NaHCO3��������NaHCO3���գ���Na2CO3���壮����NaHCO3�������������ͼ����֪������35��NH4HCO3��ʼ�ֽ⣩��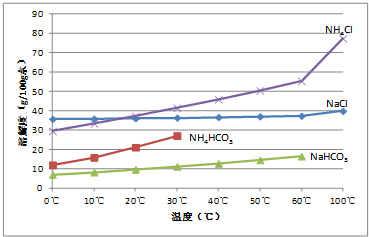
��1���ڱ���NaCl��Һ�з���������ϸ��NH4HCO3���壬���£����á����ˣ���NaHCO3���壮��Ӧ�¶ȷ�ΧӦ������
��2��������ˮϴ��NaHCO3�����Ŀ����
��3�����˺�õ���ĸҺ�к���
��4����ʹĸҺ�е�NH4Cl�ᾧ���������в��д�NaHCO3�����Բ�ȡ�Ĵ�ʩ��
a������ĥϸ��NH4HCO3���� b������ĥϸ��NaCl����
c���¶ȿ�����0��10��d���¶ȿ�����100������
��5�����Դ����Ʒ��NaHCO3�����ķ����ǣ���ȡ������ƷW g��������ˮ�ܽ⣬��1-2�η�̪����c mol/L�ı�HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ����Ӧ�����ӷ���ʽΪ
��������1��̼�������̼�����Ƶ��ܽ����30��ʱ���ϴ����¶ȹ��ͣ���Ӧ���ʽ���������35��ʱNH4HCO3��ʼ�ֽ⣬�ʷ�Ӧ�¶ȿ�����30-35��֮��Ϊ�ˣ���Ϊ���Ƶ��¶���100�����£�ʹ�¶ȱ���һ���㶨������ˮԡ���������ƣ�
��2��δ��Ӧ����Ȼ��ơ�̼����識����ɵ��Ȼ�炙ḽ����̼�����ƾ�����棻
��3������̼�����ƺ����ҺΪ̼�����Ƶı�����Һ������ĸҺ����δ��Ӧ����Ȼ��ƺ�̼���ⰱ�������ɵ��Ȼ�狀�û��������̼�����ƣ�
��4�������ܽ�ƽ��֪ʶ���ܽ�Ƚ��з�����
��5����ʼ����CO32-+H+=HCO3-���ӷ�̪��ָʾ������Һ�ɺ�ɫ����ɫ��˵����Ӧ���յ㣬�ټӼ���ָʾ����ָʾHCO3-+H+=CO2+H2O��Ӧ���յ㣬�ɷ�Ӧ��֪����Һ��CO32-��ȫ��Ӧ�����������2V1 mL����Ʒ�к��е�̼�����Ʒ�Ӧ�����������Ϊ��V2-2V1 ��mL��������Ʒ��̼������������������ʵ�������������̼�����Ƶ���������Ϊ��
��2��δ��Ӧ����Ȼ��ơ�̼����識����ɵ��Ȼ�炙ḽ����̼�����ƾ�����棻
��3������̼�����ƺ����ҺΪ̼�����Ƶı�����Һ������ĸҺ����δ��Ӧ����Ȼ��ƺ�̼���ⰱ�������ɵ��Ȼ�狀�û��������̼�����ƣ�
��4�������ܽ�ƽ��֪ʶ���ܽ�Ƚ��з�����
��5����ʼ����CO32-+H+=HCO3-���ӷ�̪��ָʾ������Һ�ɺ�ɫ����ɫ��˵����Ӧ���յ㣬�ټӼ���ָʾ����ָʾHCO3-+H+=CO2+H2O��Ӧ���յ㣬�ɷ�Ӧ��֪����Һ��CO32-��ȫ��Ӧ�����������2V1 mL����Ʒ�к��е�̼�����Ʒ�Ӧ�����������Ϊ��V2-2V1 ��mL��������Ʒ��̼������������������ʵ�������������̼�����Ƶ���������Ϊ��
����⣺��1��̼�������̼�����Ƶ��ܽ����30��ʱ���ϴ�������̼���������������¶ȹ��ͣ���Ӧ���ʽ���������35��ʱNH4HCO3��ʼ�ֽ⣬�ʷ�Ӧ�¶ȿ�����30��35��֮��Ϊ�ˣ���Ϊ���Ƶ��¶���100�����£�ʹ�¶ȱ���һ���㶨������ˮԡ���������ƣ�
�ʴ�Ϊ��30��35�棻ˮԡ���ȣ�
��2��δ��Ӧ����Ȼ��ơ�̼����識����ɵ��Ȼ�炙ḽ����̼�����ƾ�����棬������ˮϴ��NaHCO3�����Ŀ����Ϊ��ȥ�����ھ�������NH4+��Cl-��
�ʴ�Ϊ����ȥ�����ھ�������NH4+��Cl-��
��3�����˳�ȥ������̼�����ƣ���Һ�л��в���̼������δ��������̼�����Ƶı�����Һ�������ܽ�����Һ��δ������NaCl��NH4Cl��NH4HCO3�����õ�ĸҺ��Ҫ�ɷ�ΪNaHCO3��NaCl��NH4Cl��NH4HCO3��
�ʴ�Ϊ��NaHCO3��NaCl��NH4Cl��NH4HCO3��NaHCO3��
��4��a������ĥϸ��NH4HCO3���壬������Һ��HCO3-Ũ�ȣ��ܽ�ƽ��������̼�����Ʒ����ƶ�������̼�����ƣ���a����
b������ĥϸ��NaCl���壬������ҺCl-Ũ�ȵ�һ���̶ȣ��ܽ�ƽ���������Ȼ�立����ƶ��������Ȼ�泥���b��ȷ��
c���¶ȿ�����0��10��ʱ���Ȼ�淋��ܽ��С���Ȼ��ƣ��������Ȼ�淋���������ĸҺ��̼������Ϊ������Һ���¶Ƚ��ͻ�����̼�����ƣ���c����
d���¶���100�����ң��Ȼ�淋��ܽ�Ⱥܴ������Ȼ����������d����
�ʴ�Ϊ��b��
��5����ʼ����CO32-+H+=HCO3-���ӷ�̪��ָʾ������Һ�ɺ�ɫ����ɫ��˵����Ӧ���յ㣬�ټӼ���ָʾ����ָʾHCO3-+H+=CO2+H2O��Ӧ���յ㣬�ɷ�Ӧ��֪����Һ��CO32-��ȫ��Ӧ�����������2V1 mL����Ʒ�к��е�̼�����Ʒ�Ӧ�����������Ϊ��V2-2V1 ��mL������Ʒ��̼������������������ʵ���=cmol/L����V2-2V1����10-3L=c����V2-2V1����10-3mol���ɷ���ʽ��֪����Ʒ��n��NaHCO3��=c����V2-2V1����10-3mol������Ʒ��̼�����Ƶ���������=
��100%=
%��
�ʴ�Ϊ��CO32-+H+=HCO3-����V2-2V1 ����
%��
�ʴ�Ϊ��30��35�棻ˮԡ���ȣ�
��2��δ��Ӧ����Ȼ��ơ�̼����識����ɵ��Ȼ�炙ḽ����̼�����ƾ�����棬������ˮϴ��NaHCO3�����Ŀ����Ϊ��ȥ�����ھ�������NH4+��Cl-��
�ʴ�Ϊ����ȥ�����ھ�������NH4+��Cl-��
��3�����˳�ȥ������̼�����ƣ���Һ�л��в���̼������δ��������̼�����Ƶı�����Һ�������ܽ�����Һ��δ������NaCl��NH4Cl��NH4HCO3�����õ�ĸҺ��Ҫ�ɷ�ΪNaHCO3��NaCl��NH4Cl��NH4HCO3��
�ʴ�Ϊ��NaHCO3��NaCl��NH4Cl��NH4HCO3��NaHCO3��
��4��a������ĥϸ��NH4HCO3���壬������Һ��HCO3-Ũ�ȣ��ܽ�ƽ��������̼�����Ʒ����ƶ�������̼�����ƣ���a����
b������ĥϸ��NaCl���壬������ҺCl-Ũ�ȵ�һ���̶ȣ��ܽ�ƽ���������Ȼ�立����ƶ��������Ȼ�泥���b��ȷ��
c���¶ȿ�����0��10��ʱ���Ȼ�淋��ܽ��С���Ȼ��ƣ��������Ȼ�淋���������ĸҺ��̼������Ϊ������Һ���¶Ƚ��ͻ�����̼�����ƣ���c����
d���¶���100�����ң��Ȼ�淋��ܽ�Ⱥܴ������Ȼ����������d����
�ʴ�Ϊ��b��
��5����ʼ����CO32-+H+=HCO3-���ӷ�̪��ָʾ������Һ�ɺ�ɫ����ɫ��˵����Ӧ���յ㣬�ټӼ���ָʾ����ָʾHCO3-+H+=CO2+H2O��Ӧ���յ㣬�ɷ�Ӧ��֪����Һ��CO32-��ȫ��Ӧ�����������2V1 mL����Ʒ�к��е�̼�����Ʒ�Ӧ�����������Ϊ��V2-2V1 ��mL������Ʒ��̼������������������ʵ���=cmol/L����V2-2V1����10-3L=c����V2-2V1����10-3mol���ɷ���ʽ��֪����Ʒ��n��NaHCO3��=c����V2-2V1����10-3mol������Ʒ��̼�����Ƶ���������=
| c��(V2-2V1)��10-3mol��84g/mol |
| W g |
| 8.4c(V2-2V1) |
| W |
�ʴ�Ϊ��CO32-+H+=HCO3-����V2-2V1 ����
| 8.4c(V2-2V1) |
| W |
���������⿼������Ƽ���ܽ�ȡ����ʵķ����ᴿ�����ʺ����ⶨ���ζ�ԭ��Ӧ�õȣ��ѶȽϴ��ؿ���ѧ�����ܽ�����ߵķ������ã�ע����ݴ��ڵ������ж�ĸҺ�п��ܴ��ڵ����ʣ���Ŀ�漰���ʵ��ܽ�ƽ�����⣬����ܽ�ƽ����н��

��ϰ��ϵ�д�
�����Ŀ
ij��ȤС����Ʋ�����������ʵ����̽��Cl2��Ư�۵��Ʊ����й����ʣ�
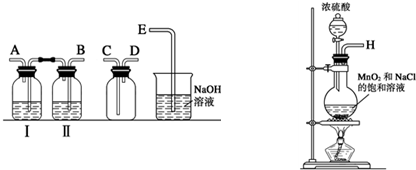
��1��ʵ����������ͼװ���Ʊ����﴿�����������밴������������������ķ��������������ӣ�H�� �� �� �� �� �����ƿ���е��Լ�Ϊ ��
��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ�� ��
��3��ijѧ��������ʵ���һ��̽��SO2��Ư�۾��ķ�Ӧ����Ư�۾��� ��
��4����ͬѧ�Ʋ�����i����״��������СҺ���γɣ���������ʵ����Խ�һ����֤��
a����ʪ��ĵ⻯�ص�����ֽ������״��ޱ仯��
b���Ѽ���״����ữ��AgNO3��Һ���飬������ɫ������
ʵ��a��Ŀ���� ��
��5����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾���ijЩ�ɷ�֮�䷢����Ӧ�������ʵ�鷽������һ��ȷ�����ֿ����ԣ�����Ϊ ��
��6���û�ѧ����ʽ���������л���ɫ��ȥ��ԭ�� ��������ɫ����Һ���Ƿ���Cl-�ķ����ǣ� ��
��1��ʵ����������ͼװ���Ʊ����﴿�����������밴������������������ķ��������������ӣ�H��

��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��
��3��ijѧ��������ʵ���һ��̽��SO2��Ư�۾��ķ�Ӧ����Ư�۾���
| ���� | ���� |
| ȡ4gƯ�۾����壬����100mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
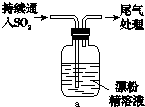 |
����Һ���Ϸ�������״�� �����Ժ��ֻ��ǣ���Һ��Ϊ����ɫ �����Ժ���������ɫ����������ɫ��ȥ |
a����ʪ��ĵ⻯�ص�����ֽ������״��ޱ仯��
b���Ѽ���״����ữ��AgNO3��Һ���飬������ɫ������
ʵ��a��Ŀ����
��5����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾���ijЩ�ɷ�֮�䷢����Ӧ�������ʵ�鷽������һ��ȷ�����ֿ����ԣ�����Ϊ
��6���û�ѧ����ʽ���������л���ɫ��ȥ��ԭ��