��Ŀ����
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
��1��W��Ԫ�����ڱ��е�λ����______��Z2Y�ĵ���ʽ��______��
��2����ҵ�ϳ�XQ3�Ƿ��ȷ�Ӧ�����д�ʩ�У����ܼӿ췴Ӧ���ʣ��������ԭ��ת���ʵ���______��
a�������¶� b���������
c����XQ3��ʱ�����ȥ d������Ӧ��ϵ��ѹǿ
��3��2.24L����״����XQ3��200mL 1mol/L QXY3��Һ���պ�������Һ������Ũ�ȴӴ�С��˳����______��
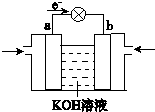
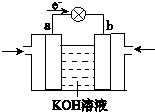
��4��WQ4Y��Y2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��a���ĵ缫��Ӧʽ��______��
��5����֪��W��s��+Y2 ��g��=WY2��g����H=-393.5kJ/mol WY��g��+
Y2 ��g��=WY2��g����H=-283.0kJ/mol
24g W��һ������Y2��Ӧ���ų�����362.5kJ�����ò�������ʵ���֮����______��
��6��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��______��
��1��W��Ԫ�����ڱ��е�λ����______��Z2Y�ĵ���ʽ��______��
��2����ҵ�ϳ�XQ3�Ƿ��ȷ�Ӧ�����д�ʩ�У����ܼӿ췴Ӧ���ʣ��������ԭ��ת���ʵ���______��
a�������¶� b���������
c����XQ3��ʱ�����ȥ d������Ӧ��ϵ��ѹǿ
��3��2.24L����״����XQ3��200mL 1mol/L QXY3��Һ���պ�������Һ������Ũ�ȴӴ�С��˳����______��
��4��WQ4Y��Y2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��a���ĵ缫��Ӧʽ��______��

��5����֪��W��s��+Y2 ��g��=WY2��g����H=-393.5kJ/mol WY��g��+
| 1 |
| 2 |
24g W��һ������Y2��Ӧ���ų�����362.5kJ�����ò�������ʵ���֮����______��
��6��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��______��
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ��������ų��Ĵ�����Ⱦ�ﳣ������CO��NO��W��X��Yԭ����������������WΪCԪ�أ�XΪNԪ�أ�YΪOԪ�أ�Q��W��ɵĻ������Ǿ�������ЧӦ�����壬ΪCH4���壬��QΪHԪ�أ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����ӦΪNa20��Na2O2���ֻ������ZΪNaԪ�أ���QΪHԪ�أ�WΪCԪ�أ�XΪNԪ�أ�YΪOԪ�أ�ZΪNaԪ�أ�
��1��WΪCԪ�أ���2�����Ӳ㣬����������Ϊ4��λ�����ڱ��ڶ�����IVA�壻Z2Y��Na2O�����������������ӹ��ɣ�����ʽ��
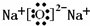
��
�ʴ�Ϊ���ڶ�����IVA�壻
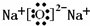
��
��2����ҵ�ϳ�NH3�������С�ķ��ȷ�Ӧ�����д�ʩ�У����ܼӿ췴Ӧ���ʣ��������ԭ��ת���ʵ���
a�������¶ȣ���Ӧ�ӿ죬ƽ�������ȷ����ƶ��������淴Ӧ�ƶ���ԭ��ת���ʽ��ͣ���a�����ϣ�
b���������������Ӧ���ʣ�ƽ�ⲻ�ƶ���ԭ��ת���ʲ��䣬��b�����ϣ�
c����XQ3��ʱ�����ȥ����Ӧ���ʼ�С��ƽ��������Ӧ�ƶ���ԭ��ת��������c�����ϣ�
d������Ӧ��ϵ��ѹǿ����Ӧ���ʼӿ죬ƽ��������Ӧ�ƶ���ԭ��ת��������d���ϣ�
��ѡ��d��
��3��2.24L����״����NH3Ϊ0.1mol��200mL1mol/L HNO3��Һ����HNO30.2mol��������������Һ���գ���Һ�൱�ں���0.1molHNO3��0.1molNH4NO3��ϣ�笠�����ˮ�ⲻ����Һ�����ԣ�������Һ������Ũ�ȴӴ�С��˳����c��NO3-����c��H+����c��NH4+����c��OH-����
�ʴ�Ϊ��c��NO3-����c��H+����c��NH4+����c��OH-����
��4����ͼ��֪�����Ӵ�a��������a��Ϊԭ��ظ�������������������Ӧ��CH4O�ڸ����Ϸŵ磬�缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��
��5����֪����C��s��+O2 ��g��=CO2��g����H=-393.5kJ/mol��
��CO��g��+
O2 ��g��=CO2��g����H=-283.0kJ/mol��
�ɢ�-�ڵã�C��s��+
O2 ��g��=CO��g����H=-110kJ/mol��
24gC�����ʵ���Ϊ2mol����һ������O2��Ӧ����ֻ���ɶ�����̼���ų�����Ϊ393.5kJ/mol��2mol=787kJ����ֻ����һ����̼���ų�����Ϊ110kJ/mol��2mol=220kJ��ʵ�ʷų�����362.5kJ�������ɶ�����̼��һ����̼�������ɶ�����̼xmol��һ����̼ymol������x+y=2��393.5x+110y=362.5�����x=0.5��y=1.5������n��CO2����n��CO��=1��3��
�ʴ�Ϊ��n��CO2����n��CO��=1��3��
��6��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�û�����ΪNa3N���÷�Ӧ�Ļ�ѧ����ʽ��Na3N+4H2O=3NaOH+NH3?H2O��
�ʴ�Ϊ��Na3N+4H2O=3NaOH+NH3?H2O��
��1��WΪCԪ�أ���2�����Ӳ㣬����������Ϊ4��λ�����ڱ��ڶ�����IVA�壻Z2Y��Na2O�����������������ӹ��ɣ�����ʽ��
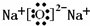
��
�ʴ�Ϊ���ڶ�����IVA�壻
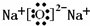
��
��2����ҵ�ϳ�NH3�������С�ķ��ȷ�Ӧ�����д�ʩ�У����ܼӿ췴Ӧ���ʣ��������ԭ��ת���ʵ���
a�������¶ȣ���Ӧ�ӿ죬ƽ�������ȷ����ƶ��������淴Ӧ�ƶ���ԭ��ת���ʽ��ͣ���a�����ϣ�
b���������������Ӧ���ʣ�ƽ�ⲻ�ƶ���ԭ��ת���ʲ��䣬��b�����ϣ�
c����XQ3��ʱ�����ȥ����Ӧ���ʼ�С��ƽ��������Ӧ�ƶ���ԭ��ת��������c�����ϣ�
d������Ӧ��ϵ��ѹǿ����Ӧ���ʼӿ죬ƽ��������Ӧ�ƶ���ԭ��ת��������d���ϣ�
��ѡ��d��
��3��2.24L����״����NH3Ϊ0.1mol��200mL1mol/L HNO3��Һ����HNO30.2mol��������������Һ���գ���Һ�൱�ں���0.1molHNO3��0.1molNH4NO3��ϣ�笠�����ˮ�ⲻ����Һ�����ԣ�������Һ������Ũ�ȴӴ�С��˳����c��NO3-����c��H+����c��NH4+����c��OH-����
�ʴ�Ϊ��c��NO3-����c��H+����c��NH4+����c��OH-����
��4����ͼ��֪�����Ӵ�a��������a��Ϊԭ��ظ�������������������Ӧ��CH4O�ڸ����Ϸŵ磬�缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��
��5����֪����C��s��+O2 ��g��=CO2��g����H=-393.5kJ/mol��
��CO��g��+
| 1 |
| 2 |
�ɢ�-�ڵã�C��s��+
| 1 |
| 2 |
24gC�����ʵ���Ϊ2mol����һ������O2��Ӧ����ֻ���ɶ�����̼���ų�����Ϊ393.5kJ/mol��2mol=787kJ����ֻ����һ����̼���ų�����Ϊ110kJ/mol��2mol=220kJ��ʵ�ʷų�����362.5kJ�������ɶ�����̼��һ����̼�������ɶ�����̼xmol��һ����̼ymol������x+y=2��393.5x+110y=362.5�����x=0.5��y=1.5������n��CO2����n��CO��=1��3��
�ʴ�Ϊ��n��CO2����n��CO��=1��3��
��6��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�û�����ΪNa3N���÷�Ӧ�Ļ�ѧ����ʽ��Na3N+4H2O=3NaOH+NH3?H2O��
�ʴ�Ϊ��Na3N+4H2O=3NaOH+NH3?H2O��

��ϰ��ϵ�д�
�����Ŀ
Q��W��X��Y��Z��ԭ��������������Ķ�����Ԫ�أ�X����ɫ��Ӧ�ʻ�ɫ��QԪ�ص�ԭ�����������������ڲ��������2����W��Z������������ͬ��Z�ĺ˵������W��2����Ԫ��Y�ĺϽ����ճ�������ʹ�ù㷺�Ľ�������֮һ������˵����ȷ���ǣ�������
| A���⻯���ȶ��ԣ�Z��W | B��ԭ�Ӱ뾶�Ĵ�С˳��rX��rY��rQ��rW | C��Ԫ��Q��Z���γ�QZ2�͵Ĺ��ۻ����� | D��X��Y������������ˮ����֮�䲻�ܷ�����Ӧ |
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
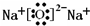
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮