��Ŀ����
������Һ�и�����Ũ�ȹ�ϵ��ȷ����
A. 0.2 mol/L��CH3COOH��Һ��0.1 mol/L CH3COOH��Һ��c(H+)֮��Ϊ2:1
B. 0.1 mol/L NaHCO3��Һ�У�c(Na+) = 2c(CO32��) + c(HCO3��) + c(H2CO3)
C. 0.1 mol/L ��NaHA��Һ����pH = 4����c(HA��)�� c(A2��)�� c(H2A)
D. ֻ��Na+��CH3COO����H+��OH����������Һ�У�c(H+)��c(CH3COO��)��c(Na+)��c(OH��)
C

��ϰ��ϵ�д�
 �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ
 W��X��Y��Z��ԭ������������������ֶ�����Ԫ�أ���֪��W��һ��ԭ�ӵ�ԭ�Ӻ���û�����ӣ�Y��X���ڣ�Y��ZҲ���ڣ�X��Y��Z����Ԫ��ԭ�ӵ�����������֮��Ϊ3����������Ԫ��Z�ڵؿ��к�����ߣ��Իش����и��⣺
W��X��Y��Z��ԭ������������������ֶ�����Ԫ�أ���֪��W��һ��ԭ�ӵ�ԭ�Ӻ���û�����ӣ�Y��X���ڣ�Y��ZҲ���ڣ�X��Y��Z����Ԫ��ԭ�ӵ�����������֮��Ϊ3����������Ԫ��Z�ڵؿ��к�����ߣ��Իش����и��⣺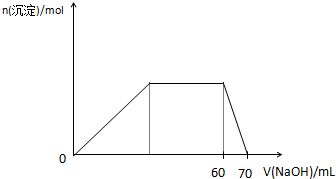 ��2011?����ģ�⣩X��Y��Z��M��N��K���ɶ�����Ԫ�ع��ɵ���������X��Y��Z�������ӣ�M��N�����Է��ӣ�K�������ӣ����Ǿ������нṹ�ص�����ʣ�
��2011?����ģ�⣩X��Y��Z��M��N��K���ɶ�����Ԫ�ع��ɵ���������X��Y��Z�������ӣ�M��N�����Է��ӣ�K�������ӣ����Ǿ������нṹ�ص�����ʣ�

