��Ŀ����
��ҵ�������̿�Ϊԭ�ϣ��������������Ʊ��ߴ��������̵��������£�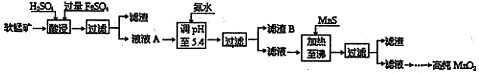
ij���̿����Ҫ�ɷ�ΪMnO2��������Si��16��27%����Fe��5��86%����Al��3��42%����Zn��2��68%����Cu��0��86%����Ԫ�صĻ��������������������������������ʽ��ȫ����ʱ��Һ��pH���±���
�ش��������⣺
��1���������������������½�MnO2��ԭΪMnSO4�����ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ ��
��2������pHʱ������������ԭ���ǣ� �������ϣ�����pH����СֵΪ ������B����Ҫ�ɷ��� ��
��3������MnS��Ŀ���dz�ȥ ���ʡ�
��4������п�̵���У�MnO2����ĵ缫��Ӧ����ʽΪ �����շϾɼ����̵���е��̣������̻�����м���һ������ϡ���ᡢϡ���ᣬ�����Ͻ�����������Ϊֹ������Ҫ��ӦΪ��2MnO��OH��+MnO2 +2H2C2O4 +3H2SO4=3MnSO4 +4CO2 ��+6H2O���÷������ŵ��� ��
��1��MnO2��2Fe2����4H��=Mn2����2Fe3����2H2O
��2��ʹAl(OH)3�ܽ⡢ʹMn2������ 5.2 Fe(OH)3�� Al(OH)3
��3��Cu2�� Zn2��
��4��MnO2��H2O��e��=MnOOH��OH��(��2MnO2��H2O��2e��=Mn2O3��2OH��) �������̼�����CO2��H2O��Ӱ��MnSO4���ȣ���Ӧ�������ж��к��������ɣ�����ɶ�����Ⱦ��������Դ���ȡ�
����

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д���ѧѧ���еĻ�ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺
��1�������£�ȡpH��2������ʹ�����Һ��100 mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ���________���A����B�������������вμӷ�Ӧ��Zn������Ϊm1��������Һ�вμӷ�Ӧ��Zn������Ϊm2����m1________m2��ѡ���������������������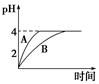
��2����֪������Cu��OH��2��Ksp��2��10��20����֪������ijCuSO4��Һ��c��Cu2������0.02 mol��L��1�����Ҫ����Cu��OH��2��������Ӧ������ҺpH����________��Ҫʹ0.2 mol��L��1��CuSO4��Һ��Cu2��������Ϊ��ȫ��ʹCu2��Ũ�Ƚ���ԭ����ǧ��֮һ������Ӧ����Һ���NaOH��Һ��ʹ��ҺpHΪ________��
��3��10 ��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
| �¶�/�� | 10 | 20 | 30 | ������к���ȴ��50 �� |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
�ڷ�������ҺpH�����ԭ��ʱ����ͬѧ��Ϊ�������¶�HCO3-��ˮ��̶��������£���ͬѧ��Ϊ����Һ�������¶�NaHCO3���ȷֽ�����Na2CO3��CO32-ˮ��̶ȴ���HCO3-���¡��������һ����ʵ�鷽����������λͬѧ��˵�������У���������������ͽ��ۣ�________________________________________________________________________��
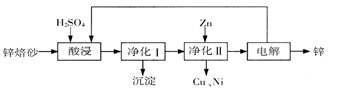
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�
��֪��Zn���仯�����������Al���仯������������ƣ���ش��������⣺
(1)��NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
(3)����ҺB��ȡFe3O4�������ӵĹ����У������ͨ��N2����ԭ����
_______________________________________________________________
(4)Fe3O4���������ܷ��ü�ѹ���˷�ʵ�ֹ�Һ���룿__________(��ܡ����ܡ�)��������________________________________��
(5)���ظ���ط�(һ��������ԭ�ζ���)�ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.010 00 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ________ g K2Cr2O7(����4λ��Ч���֣���֪MK2Cr2O7��294.0 g��mol��1)�����Ƹñ���Һʱ�����������в���Ҫ�õ�����________(�ñ�ű�ʾ)��
�ٵ�����ƽ�����ձ�������Ͳ���ܲ�����
������ƿ ��ͷ�ιܡ�����Һ��
(6) �ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ�����________(�ƫ����ƫС�����䡱)��
����Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��
�����ᡢ�ڴ�����Һ��������������Һ�����Ȼ����Һ���ݴ������Һ�����������Һ�������������Һ���ఱˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H��Ũ���ɴ�С��˳���ǣ�����ţ�________��
��2���ܡ��ݡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳���ǣ�����ţ�________��
��3�����ۺܵ͢������Ϻ��Һ�и�����Ũ�ȹ�ϵ��ȷ����________��
| A��c��Na������c��Cl������c��OH������c��NH4+�� |
| B��c��Na������0.1 mol��L��1 |
| C��c��Na������c��NH4+����c��Cl������c��OH���� |
| D��c��H������c��OH���� |
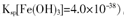 ��
�� HSO3-��H���ĵ��볣��Ka��1��10��2mol��L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb��________mol��L��1������NaHSO3��Һ�м���������I2������Һ��
HSO3-��H���ĵ��볣��Ka��1��10��2mol��L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb��________mol��L��1������NaHSO3��Һ�м���������I2������Һ��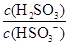 ��________(���������С�����䡱)��
��________(���������С�����䡱)��