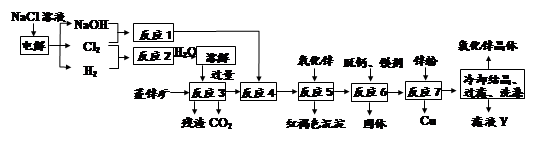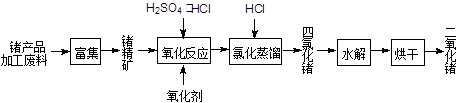��Ŀ����
ú����Ҫ����Դ��Ҳ������������Ʒ����Ҫԭ�ϡ�������ѧ֪ʶ������������⣺
��1��ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊ �� ��
��2����úȼ��ǰ���ú������������ú��ij����������ԭ������ͼ��ʾ��
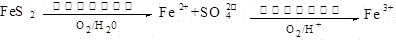
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
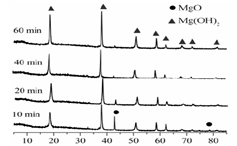
��3����ҵú����õ��IJ�Ʒ�н�̿�� ��
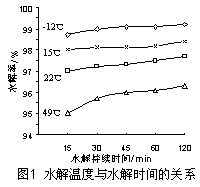
��4����ҵ����Ҫ���ð��������������ᣬ��ͼ�ǰ��������백������������������ȵĹ�ϵ������ֱ�߱�ʾ��Ӧ������ֵ�����߱�ʾ����ʵ����������������ʴﵽ100���������Ϧã�n(O2)��n(NH3)���� ��ʵ������Ҫ����ֵά����1.7��2.2֮�䣬 ԭ���� ��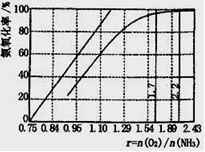
��1��ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊ �� ��
��2����úȼ��ǰ���ú������������ú��ij����������ԭ������ͼ��ʾ��
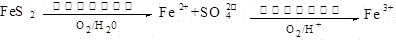
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
��3����ҵú����õ��IJ�Ʒ�н�̿�� ��
��4����ҵ����Ҫ���ð��������������ᣬ��ͼ�ǰ��������백������������������ȵĹ�ϵ������ֱ�߱�ʾ��Ӧ������ֵ�����߱�ʾ����ʵ����������������ʴﵽ100���������Ϧã�n(O2)��n(NH3)���� ��ʵ������Ҫ����ֵά����1.7��2.2֮�䣬 ԭ���� ��

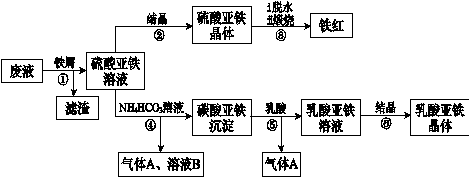
����8�֣���1��ֱ��Һ�����������Һ��������ȫ�Ը�1�֣�
��2��2FeS2��7O2��2H2O 4H+��2Fe2+��4SO42-��2�֣� 4Fe2+��O2��4H+
4H+��2Fe2+��4SO42-��2�֣� 4Fe2+��O2��4H+ 4Fe3+��2H2O��2�֣�
4Fe3+��2H2O��2�֣�
��3����¯ú�����ְ�ˮ��ú���ͣ�ȫ�Ը�1�֣�
��4��1.25��1�֣��� O2̫�ٲ�����NH3��ת������ֵΪ2.2ʱNH3�������ѽ�100%��1�֣�
��2��2FeS2��7O2��2H2O
 4H+��2Fe2+��4SO42-��2�֣� 4Fe2+��O2��4H+
4H+��2Fe2+��4SO42-��2�֣� 4Fe2+��O2��4H+ 4Fe3+��2H2O��2�֣�
4Fe3+��2H2O��2�֣���3����¯ú�����ְ�ˮ��ú���ͣ�ȫ�Ը�1�֣�
��4��1.25��1�֣��� O2̫�ٲ�����NH3��ת������ֵΪ2.2ʱNH3�������ѽ�100%��1�֣�
�����������1��ú��Һ����Ϊֱ��Һ���ͼ��Һ��������
��2����һ����Ӧ�з�Ӧ����FeS2��O2��H2O����������Fe2+��SO42-����Ӧ��SԪ�صĻ��ϼ۴ӣ�1�����ߵ���6�ۣ�ʧȥ7�����ӡ���Ԫ�صĻ��ϼ۴�0�۽��͵���2�ۣ����Ը��ݻ��ϼ�������������Լ�ԭ���غ��֪���÷�Ӧ�����ӷ���ʽΪ2FeS2��7O2��2H2O
 4H+��2Fe2+��4SO42-��Fe2+���л�ԭ�ԣ��ɱ���������ΪFe3+��ͬ�����ݻ��ϼ�������������Լ�ԭ���غ��֪���÷�Ӧ�����ӷ���ʽΪ4Fe2+��O2��4H+
4H+��2Fe2+��4SO42-��Fe2+���л�ԭ�ԣ��ɱ���������ΪFe3+��ͬ�����ݻ��ϼ�������������Լ�ԭ���غ��֪���÷�Ӧ�����ӷ���ʽΪ4Fe2+��O2��4H+ 4Fe3+��2H2O��
4Fe3+��2H2O����3����ҵú����õ��IJ�Ʒ�н�̿����¯ú�����ְ�ˮ��ú���͡�
��4�����ݰ������Ļ�ѧ����ʽ4NH3+5O2
 4NO+6H2O��֪���������ʴﵽ100%�������Ϧ�{n��O2��:n��NH3��}��5:4��1.25�������ڷ�Ӧ��O2Ũ��̫�ٲ�����NH3��ת�������ڦ�{n��O2��/n��NH3��ֵΪ2.2ʱNH3�������ѽ�100%������ʵ������Ҫ����ֵά����1.7��2.2֮�䡣
4NO+6H2O��֪���������ʴﵽ100%�������Ϧ�{n��O2��:n��NH3��}��5:4��1.25�������ڷ�Ӧ��O2Ũ��̫�ٲ�����NH3��ת�������ڦ�{n��O2��/n��NH3��ֵΪ2.2ʱNH3�������ѽ�100%������ʵ������Ҫ����ֵά����1.7��2.2֮�䡣
��ϰ��ϵ�д�
�����Ŀ