��Ŀ����
(7��)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N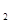 H
H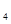 ��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��1����Ӧ���Ȼ�ѧ����ʽΪ ��
(2)����֪H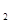 0(I)=H
0(I)=H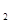 0(g)����H=+44 kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������
KJ��
0(g)����H=+44 kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������
KJ��
(3)�˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
(4)��֪N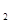 (g)+2O
(g)+2O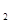 (g)=2N0
(g)=2N0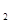 (g)
��H=+67.7kJ��mol
(g)
��H=+67.7kJ��mol
N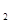 H
H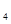 (g)+0
(g)+0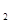 (g)=N
(g)=N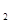 (g)+2H
(g)+2H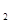 0(g)
��H=-534kJ��mol
0(g)
��H=-534kJ��mol
������N0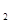 ��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ
��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ
���𰸡�
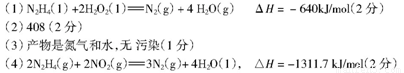
��������

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
�����Ŀ
 C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������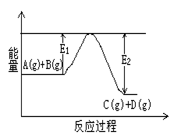
 O���Ȼ�ѧ����ʽΪ ��
O���Ȼ�ѧ����ʽΪ �� ����Һ̬˫��ˮ��H2O2����
����Һ̬˫��ˮ��H2O2����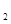 0(I)=H
0(I)=H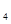 (g)+0
(g)+0 H
H ��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ�������� g)=N
g)=N