��Ŀ����
��X��Y��Z 3��Ԫ�أ���֪X��̬�⻯��Ļ�ѧʽΪH2X������̬�⻯���ʽ����X���γ�����������ʽ��֮��Ϊ17��40��Xԭ�Ӻ���������������������ͬ�ģ�Y��X�γ����ӻ�����Ļ�ѧʽΪY2X��Y�������ӵ��Ӳ�ṹ������ͬ��Z��X��ԭ�ӵ��Ӳ�����ͬ������̬������˫ԭ�ӷ��ӣ���ԭ�ӹ���1�Ե��ӡ�
(1)д����Ԫ�ص�Ԫ�ط��ţ�X___________��Y___________��Z___________��
(2)X�����ԭ������Ϊ___________��
(3)X�����ӵ���ʽΪ___________��X����Ļ�����ĵ���ʽΪ___________��
(4)Y�����ڿ�����ȼ�յĻ�ѧ����ʽΪ______________________���û�������ˮ��Ӧ�Ļ�ѧ����ʽΪ______________________���˷�Ӧ�����ӷ���ʽΪ______________________��
(5)Z�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(6)Z2�ĵ���ʽΪ___________��Z���ʵ�ˮ��Һ��Y2X��Ӧ��������___________���仯ѧ����ʽΪ(��������ת��)___________________���˷�Ӧ�����ӷ���ʽΪ___________________�������˷�Ӧ���õ��Ľ�����___________��ԭ����_____________________��
(1)S Na Cl
(2)32
![]()
(4)2Na+O2![]() Na2O2 2Na2O2+2H2O====4NaOH+O2��
Na2O2 2Na2O2+2H2O====4NaOH+O2��
2Na2O2+2H2O====4Na++4OH-+O2��
(5)Cl2+H2O====HCl+HClO
(6)![]() ��Һ�����
��Һ�����
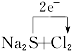 ====2NaCl+S�� S2-+Cl2====S��+2Cl-
====2NaCl+S�� S2-+Cl2====S��+2Cl-
Cl2�ķǽ����Ա�Sǿ(Cl2>S) Cl�����7e-��S�����6e-,rCl<rS��Cl�õ�������ǿ
����:
��֪X����̬�⻯��Ļ�ѧʽΪH2X�����Ƶ�X���������Ϊ+6���������������Ļ�ѧʽӦΪXO3������ΪH2X��XO3��ʽ��֮��Ϊ17��40����(2+x)��(x+48)=17��40�����xΪ32������Xԭ�Ӻ�������������������ȣ�����XΪ16����Ԫ�أ��ٸ���Y2X����֪YΪ+1�ۣ�����ΪY�������ӵ��Ӳ�ṹ��Ne��ͬ��ͨ��һ�Թ��õ��Ӷ��γ���̬˫ԭ�ӷ��ӣ�����֪ZΪ��Ԫ�ء�

