��Ŀ����
��1�����÷�Ӧ���ʿ�ʼʮ�ֻ�����һ��ʱ���ͻȻ�ӿ죬������Ϊ
��2�����ݴ�ԭ������������KMnO4��Һ���ⶨH2C2O4��Һ��Ũ�ȣ������������£�
��ȷ����0.10mol/L��KMnO4
�ڽ�KMnO4��Һʢ���ڵζ�����
��ȷ��ȡ25.00mL H2C2O4��Һ����ƿ��
�ܽ��еζ��ζ��յ���ʲô����
��3���������в����У���ʹ�ⶨ�� Ũ��ƫ�����
��ʢװ KMnO4��Һ�ĵζ���������ˮϴ����δ��KMnO4��Һ��ϴ
����ƿ��ʢ����������ˮ���ټӴ���Һ
��ʢװH2C2O4��Һ�ĵζ���������ˮϴ����δ��H2C2O4��Һ��ϴ
�ܵζ���۲�ζ��ܶ���ʱ�����߸��ڿ̶���
��4���ζ�ʱ���õ�ʵ���������£��Լ�������
| ʵ�������� | ����Һ���mL | ����ı�Һ�����mL�� |
| 1 | 25.00 | 28.95 |
| 2 | 25.00 | 25.05 |
| 3 | 25.00 | 24.95 |
��2��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ��յ���ɫ���жϣ���Ҫȷ����õζ�ʵ���ԭ������ʵ����������ԭ�ζ����յ�ʱKMnO4��Һǡ�ù���һ�Σ���Һ�����Ϻ�ɫ������Ҫ���ָʾ����
��3������C�����⣩�T
| C(��)��V(��) |
| V(����) |
��4����1���������ϴ���ȥ�����������������ݣ�����5H2C2O4��2MnO4-���㣮
��2��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ���KMnO4��ҺӦװ����ʽ�ζ����У���ʵ����������ԭ�ζ����յ�ʱKMnO4��Һǡ�ù���һ�Σ���Һ�����Ϻ�ɫ��30���ڲ���ɫ������Ҫ���ָʾ�����ʴ�Ϊ����ʽ����Һ����ɫ��Ϊ�Ϻ�ɫ��30���ڲ���ɫ����
��3����ʢװKMnO4��Һ�ĵζ���������ˮϴ����δ��KMnO4��Һ��ϴ��KMnO4��ҺŨ��ƫС�����V������ƫ����C�����⣩�T
| C(��)��V(��) |
| V(����) |
����ƿ��ʢ����������ˮ���ټӴ���Һ������Һ�����ʵ������䣬��V��������Ӱ�죬����C�����⣩�T
| C(��)��V(��) |
| V(����) |
��ʢװH2C2O4��Һ�ĵζ���������ˮϴ����δ��H2C2O4��Һ��ϴ��H2C2O4��ҺŨ��ƫС������Һ�����ʵ���ƫС�����V������ƫС������C�����⣩�T
| C(��)��V(��) |
| V(����) |
�ܵζ���۲�ζ��ܶ���ʱ�����߸��ڿ̶��ߣ����V������ƫС������C�����⣩�T
| C(��)��V(��) |
| V(����) |
��ѡ���٣�
��4��5H2C2O4 ��2MnO4-
5mol 2mol
n 0.1mol/L��0.025L
n=0.00625mol��
��C��H2C2O4��=
| 0.00625mol |
| 0.025L |

 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�|
�����Ȼ�ѧ����ʽ�����ӷ���ʽ�У���ȷ���ǣ� | |
| [����] | |
A�� |
����ı�ȼ���Ȧ�HΪ��890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)��2O2(g)��CO2(g)��2H2O(g)����H����890.3 kJ��mol��1 |
B�� |
500�桢30 MPa�£���0.5 mol��N2(g)��1.5 mol��H2(g)�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪ��N2(g)��3H2(g) |
C�� |
��������Һ�м������������������Һ��Al3+��2SO |
D�� |
��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ� 2MnO |
|
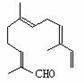
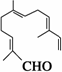
����ȵķ������пɷ���õ����½ṹ�Ļ����
�����Լ��� ������KMnO4��Һ�� ��H2/Ni�� ��Ag(NH3)2OH�� ������Cu(OH)2����Һ�� ����û����������й����Ŷ�������Ӧ���Լ��� | |
| [����] | |
A�� |
�٢� |
B�� |
�ڢ� |
C�� |
�ۢ� |
D�� |
�٢� |
|
�����Ȼ�ѧ����ʽ�����ӷ���ʽ�У���ȷ���� | |
| [����] | |
A�� |
�����ȼ����Ϊ890.3kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ�� CH4(g)��2O2(g)��CO2(g)��2H2O(g)����H����890.3kJ��mol��1 |
B�� |
500�桢30MPa�£���0.5 mol��N2��1.5 mol��H2�����ܱյ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪ�� |
C�� |
��������Һ�м������������������Һ��Al3+��2SO42����2Ba2+��4OH����2BaSO4��AlO2����2H2O |
D�� |
��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4����6H+��5H2O2��2Mn2+��5O2����8H2O |
 2NH3��g�� ��H= -38��6kJ/mol
2NH3��g�� ��H= -38��6kJ/mol