��Ŀ����
����˵��������ȷ���� ( )
A������ȱ�⣬��ͨ��ʳ�üӵ��β��⡡���� B��ȱ����ƶѪ����ͨ��ʳ����ǿ������Ԥ��
C��Ϊ�˷�ֹȣ�ݣ����˶�Ҫʹ�ú������ࡡ D��п���������࣬Ҳ������ȱ����ƶѪ
��������������п�������������ã���ʹ��������Ѫ���Ʒ����ϰ�������D����ȷ�ġ�����������ЧԤ��ȣ�ݣ������������Ӱ���ͯ�����������������Զ�ͯ������ʹ�ú������࣬C�Ǵ���ģ���ѡC��
C

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ
���õ�ť��ʽ��п��أ��ŵ�ʱ�ĵ缫��Ӧʽ�ֱ�Ϊ��
Zn����Zn+2OH--2e-��Zn��OH��2
Ag2O����Ag2O+H2O+2e-��2Ag+2OH-
������˵���в���ȷ���ǣ�������
Zn����Zn+2OH--2e-��Zn��OH��2
Ag2O����Ag2O+H2O+2e-��2Ag+2OH-
������˵���в���ȷ���ǣ�������
| A���ŵ������OH-���ʵ������� | B��ZnΪ������Ag2OΪ���� | C���������Һ�ʼ��� | D�����Ӵ�Ag2O�������õ�������Zn�� |
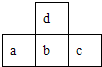 ��ͼΪԪ�����ڱ��ж����ڵ�һ���֣���֪aԭ�ӵ������������ȴ�����������3������˵���в���ȷ���ǣ�������
��ͼΪԪ�����ڱ��ж����ڵ�һ���֣���֪aԭ�ӵ������������ȴ�����������3������˵���в���ȷ���ǣ�������| A��Ԫ��c�ĺ�����һ����ǿ�� | B��Ԫ��a��d��ԭ�Ӱ뾶��a��d | C��Ԫ��b����Ȼ���д�������̬ | D��Ԫ��b��c�������Ӱ뾶��b��c |
����˵���в���ȷ���ǣ�������
| A��IBr�����У�BrΪ-1�� | B��ijԪ��ԭ�ӵļ۵����Ų�Ϊnsn-2npn+1����Ԫ��λ�ڵ�4���ڢ�A�� | C����̬18Oԭ������8���˶�״̬��ȫ��ͬ�ĵ��� | D��2p3��ʾ2p�ܼ���������� |
����˵���в���ȷ���ǣ�������
| A��Ԫ�صĵ�һ��������Ԫ�صĵ���ʧȥ�����1����������Ҫ���յ�������ͬ���ڴ�����Ԫ�صĵ�һ������������ | B��Ԫ�صĵ縺������������ͬԪ�ص�ԭ�ӶԼ��ϵ����������Ĵ�С���縺��Խ���ԭ�ӶԼ��ϵ��ӵ�������Խ�� | C��һ����˵�����ڱ������ң�Ԫ�صĵ縺��������ڱ����ϵ��£�Ԫ�صĵ縺����С | D�����ֵĵ縺�����Է��ĵ縺��Ϊ4.0��Ϊ��Ա��ó���Ԫ�صĵ縺�ԣ�ϡ������δ�ƣ� |