��Ŀ����
8�����Т�CaBr2 �ڶ������� ��Ar ��Na2SO4 �ݸɱ�������߰��� �������ʣ�������Ҫ��ش�����ţ���ѡ��©ѡ�����֣���1���������Ӿ�����Ǣ٢ܣ� ���ڷ��Ӿ�����Ǣۢݢޢߣ�
��2��������ֻ����һ�������������Ǣ٢ڢۣ�
��3�������ۻ�ʱ��Ҫ�ƻ����ۼ����Ǣڣ�
��4�������мȺ����Ӽ����ֺ����ۼ����Ǣܣ�
��5�����岻���磬��ѹ���ۻ�ʱ�ܵ�����Ǣ٢ܣ�
��6����֪��25�棬101kPa�£�lgҺ̬C8H18�����飩ȼ�����ɶ�����̼��Һ̬ˮʱ�ų�48.40kJ������д��������Ӧ���Ȼ�ѧ����ʽC8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��l����H=-5517.6KJ/mol��
���� ��1���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ�������ͨ�����Ӽ���������ϵ����ڷ��Ӿ��壻
��2��������ֻ����һ��������������˵��������Ϊԭ�Ӿ����ԭ�ӷ��ӵķ��Ӿ����ֻ�����Ӽ������Ӿ����������壻
��3��ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ���
��4���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ���
��5�����Ӿ�����岻���磬��ѹ���ۻ�ʱ�ܵ��磻
��6����������������㣬114g������ȫȼ�����ɶ�����̼��Һ̬ˮ�ų�����5517.6KJ/mol������Ȼ�ѧ����ʽ��д������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ�д����
��� �⣺��CaBr2 �������Ӿ��壬ֻ�������Ӽ���
�ڶ��������д��ڹ��ۼ�������ԭ�Ӿ��壻
��Ar������û�й��ۼ���ֻ�з��Ӽ������������ڷ��Ӿ��壻
��Na2SO4 �������Ӿ��壬�������Ӽ����ۼ���
�ݸɱ����Ӵ��ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
��������ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
�߰��״��ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
��1���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ����������Ӿ�����Ǣ٢ܣ�����ͨ�����Ӽ���������ϵ����ڷ��Ӿ��壬��ۢݢޢ����ڷ��Ӿ��壻
�ʴ�Ϊ���٢ܣ��ۢݢޢߣ�
��2��������ֻ����һ��������������˵��������Ϊԭ�Ӿ����ԭ�ӷ��ӵķ��Ӿ����ֻ�����Ӽ������Ӿ����������壬��Ϊ�٢ڢۣ��ʴ�Ϊ���٢ڢۣ�
��3��ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ�������ԭ�Ӿ����Ϊ���ڣ��ʴ�Ϊ���ڣ�
��4���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ����Ⱥ����Ӽ����ֺ����ۼ����Ǣܣ��ʴ�Ϊ���ܣ�
��5�����Ӿ�����岻���磬��ѹ���ۻ�ʱ�ܵ��磬���Թ��岻���磬��ѹ���ۻ�ʱ�ܵ�����Ǣ٢ܣ��ʴ�Ϊ���٢ܣ�
��6��25�桢101kpa�£�1gC8H18�����飩ȼ�����ɶ�����̼��Һ̬ˮʱ�ų�48.40kJ������114g������ȫȼ�����ɶ�����̼��Һ̬ˮ�ų�����5517.6KJ/mol����Ӧ���Ȼ�ѧ����ʽΪ��C8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��l����H=-5517.6KJ/mol��
�ʴ�Ϊ��C8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��l����H=-5517.6KJ/mol��
���� ���⿼���˾����д��ڵ����������Ȼ�ѧ����ʽ����д�ȣ����ݾ���Ĺ�����������������������������Ŀ�ѶȲ���

 ��������ϵ�д�
��������ϵ�д�| A�� | ��AgNO3��Һ���백ˮ����������Һ | |
| B�� | �����뱽�״���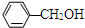 ������������һ��-CH2ԭ���ţ�������ǻ�Ϊͬϵ�� ������������һ��-CH2ԭ���ţ�������ǻ�Ϊͬϵ�� | |
| C�� | �����������£�CH3CO18OC2H5��ˮ�������CH3CO18OH��C2H5OH | |
| D�� | ���ӷ����������ǻ��Ա�����Ӱ�죬ʹ�����ϵ���ԭ�����ױ�ȡ�� |
 ���豶���������к��в��ӣ���������Ŀǰ�в����˹��ϳɵĴ���Ȼ����ܡ���Ч�ܵĿ������������ɻ�������������ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC�����������в���ȷ���ǣ�������
���豶���������к��в��ӣ���������Ŀǰ�в����˹��ϳɵĴ���Ȼ����ܡ���Ч�ܵĿ������������ɻ�������������ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC�����������в���ȷ���ǣ�������| A�� | EGC���������е�ԭ��һ�������� | |
| B�� | 1mol EGC������2molBr2����ȡ����Ӧ | |
| C�� | EGC���Է���������Ӧ��ȡ����Ӧ��1mol�����ʿ�����6molH2�����ӳɷ�Ӧ | |
| D�� | 1molEGC��3molNaǡ����ȫ��Ӧ |
| A�� | ��ʯȼ�ϵȴ�ͳ��Դ�����������ʴ�ͳ��Դ��Ϊһ����Դ������Դ���Ƕ�����Դ | |
| B�� | ���ȷ�Ӧ��Ҫ������������Ϊ���ȷ�ӦΪ���ȷ�Ӧ | |
| C�� | ����������¯�ĸ߶ȿ��Լ�������ЧӦ����CO2�ܶȽϴ� | |
| D�� | ����������¯�ĸ߶Ȳ����ܼ���CO���ŷţ���Ϊû�иı䷴Ӧ���� |
| A�� | ���³�ѹ�£�7.0g��ϩ���ϩ�Ļ�����к�����ԭ�ӵ���ĿΪNA | |
| B�� | ���³�ѹ�£�22.4 L CH4�к��е�C-H����Ϊ4NA | |
| C�� | ��0.2 mol H2SO4��Ũ����������Cu��Ӧ������SO2�ķ�����Ϊ0.1NA | |
| D�� | lmol FeCl2������������Ӧʱת�Ƶĵ�����Ϊ2NA |
| ��� | �缫���� | �������Һ | ������ָ�� ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��C��ʯī�� | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | ����������Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
��1��ʵ��1��2��Al�����ĵ缫��������������ͬ�����ͬ������ͬ������
��2����ʵ��3���������գ�
����Ϊ�������缫��Ӧʽ��2Al-6e-�T2Al3+��
��ʯīΪ�������缫��Ӧʽ��6H++6e-�T3H2����
�۵���ܷ�Ӧ����ʽ��2Al+6H+�T2Al3++3H2����
��3��ʵ��4������������������Alʧȥ���ӣ�д�����缫�ĵ缫��Ӧʽ��Al-3e-+4OH-�TAlO2-+2H2O��
��4������ʵ��5�е�����ָ��ƫ������ԭ��Al��Ũ���ᷢ���ۻ�������Zn��Ũ�����������ԭ��Ӧ��Zn��������Al���������������������������Ե�����ָ��ƫ������
��5������ʵ�����ܽ��Ӱ������ԭ������������������أ��Է���������ԭ��Ӧ�н������Ƿ���뷴Ӧ���������μӷ�Ӧ��ʧ��������������֮��������
| A�� | ���д���Fe2+����Һ��Na+��NH4+��ClO-��SO42- | |
| B�� | ���д�����ˮ����Һ��Ca2+��Mg2+��Ba2+��NO3- | |
| C�� | ���д���AlO2-����Һ��Na+��K+��NO3-��CO32- | |
| D�� | ���д���NO3-����Һ��H+��I-��SO42-��Cl- |
 ȫ��Һ�������һ�ֻ������ʳ�ѭ������Һ̬�ĵ�أ�Ŀǰ����ؼ����Ѿ��������죮
ȫ��Һ�������һ�ֻ������ʳ�ѭ������Һ̬�ĵ�أ�Ŀǰ����ؼ����Ѿ��������죮
