��Ŀ����
һ������£�������һ������ȡ300mg��500mg��������ʱ���ͻ������ж���ij�о�С���õ������ⶨ�ݲ����������εĺ������йط�Ӧ���£�2NaNO2+2H2SO4+2KI=K2SO4+I2+2H2O+Na2SO4+2NO
I2+2Na2S2O3=2NaI+Na2S4O6
ȡ1kg�ݲˣ�ե֭����ե����Һ���ռ�������ȡ�����������ƣ�ʹ�õ����ݲ�֭�е��������ζ���ΪҪ�����ƣ��ڹ��˺����Һ�м�������������Һ���Գ�ȥɫ�أ��ٴι��˺�õ���Һ��������Һϡ����1L��ȡ25.00mLϡ�ͺ����Һ�������ϡ����͵⻯����Һ��Ӧ����ѡ�ú��ʵ�ָʾ������0.05000mol?L-1Na2S2O3����Һ���еζ���ʵ�����ݼ�¼���£�
�ζ����� | 0.05000mol?L-1Na2S2O3����Һ���/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | |
| 1 | 0.00 | 20.04 |
| 2 | 0.12 | 20.08 |
| 3 | 0.05 | 20.05 |
��1����ʵ���п�ѡ�õ�ָʾ����______���ζ��յ��������______��
��2���о���ij�ʦ�����ݲ�ʱ�������������ij�֭���Լ����������ε�Σ��������Ҫ����Ϊ��֭�к��зḻ��ά����C��ά����C����______�ԣ�������ԡ���ԭ�ԡ���
��3��ͨ�������ж���ij��һ��ʳ��0.125kg�����ݲˣ��Ƿ�������ж���
��2�������������ξ��������Խ����жϣ�
��3�����ݷ�Ӧ�ҳ���ϵʽ��2NaNO2��I2��2Na2S2O3��������������Ƶ����ʵ�����������������Ƶ�������
����⣺��1����������ƺ͵ⵥ�ʵķ�Ӧ������ѡ�õ�����ָʾ��������ɫ�պ���ȥ���Ұ���Ӳ��仯���ζ�������
�ʴ�Ϊ�����ۣ���ɫ�պ���ȥ��
��2���������ƾ��������ԣ��ܹ������л�ԭ�Ե�ά����������
�ʴ�Ϊ����ԭ�ԣ�
��3�����ݷ�Ӧ��2NaNO2+2H2SO4+2KI=K2SO4+I2+2H2O+Na2SO4+2NO
I2+2Na2S2O3=2NaI+Na2S4O6 ��
���Եó���ϵʽ��2NaNO2��I2��2Na2S2O3�����Եó�n��NaNO2��=n��Na2S2O3����
���εζ����ĵı�Һ������ֱ�Ϊ��20.04mL��19.96mL��20.00mL��ƽ�����Ϊ��
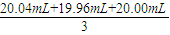 =20.00mL��
=20.00mL��ԭ1L��Һ�к��е��������Ƶ����ʵ����ǣ�
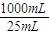 ×0.05000mol?L-1×0.025L=0.05mol��
×0.05000mol?L-1×0.025L=0.05mol��1Kg�ݲ˺��е��������Ƶ������ǣ�69g/mol×0.05mol=3.45g��
0.125Kg�ݲ˺��е��������������ǣ�
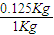 ×3.45g=0.5244g=524.4mg��300���������ж���
×3.45g=0.5244g=524.4mg��300���������ж���������524.4mg��300���������ж���
���������⿼�����ݲ����������ƺ����IJⶨ������ѧ֪ʶӦ�õ�����У������Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�һ������£�������һ������ȡ300 mg��500 mg��������ʱ���ͻ������ж���ij�о�С���õ������ⶨ�ݲ����������εĺ������йط�Ӧ���£�
2NaNO2��2H2SO4��2KI===2NO����I2��K2SO4��Na2SO4��2H2O
2Na2S2O3��I2===Na2S4O6��2NaI
ȡ1 kg�ݲˣ�ե֭����ե����Һ���ռ�������ȡ�����������ƣ�ʹ�ݲ�֭�е��������ζ���Ϊ�������ƣ��ڹ��˺����Һ�м�������������Һ���Գ�ȥɫ�أ��ٴι��˺�õ���Һ��������Һϡ����1 L��ȡ25.00 mLϡ�ͺ����Һ�������ϡ����͵⻯����Һ��Ӧ����ѡ�ú��ʵ�ָʾ������0.05000 mol��L��1Na2S2O3����Һ���еζ���ʵ�����ݼ�¼���£�
| �ζ����� | 0.050 00 mol��L��1 Na2S2O3����Һ���/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | |
| 1 | 0.00 | 20.04 |
| 2 | 0.12 | 20.08 |
| 3 | 0.05 | 20.05 |
(1)��ʵ���п�ѡ�õ�ָʾ����________���ζ��յ��������________��
(2)�о���ij�ʦ�����ݲ�ʱ�������������ij�֭���Լ����������ε�Σ��������Ҫ����Ϊ��֭�к��зḻ��ά����C��ά����C����________��(���������ԭ��)��
(3)ͨ�������ж���ij��һ��ʳ��0.125 kg�����ݲˣ��Ƿ�������ж���
һ������£�������һ������ȡ300 mg��500 mg��������ʱ���ͻ������ж���ij�о�С���õ������ⶨ�ݲ����������εĺ������йط�Ӧ���£�
2NaNO2��2H2SO4��2KI===2NO����I2��K2SO4��Na2SO4��2H2O
2Na2S2O3��I2===Na2S4O6��2NaI
ȡ1 kg�ݲˣ�ե֭����ե����Һ���ռ�������ȡ�����������ƣ�ʹ�ݲ�֭�е��������ζ���Ϊ�������ƣ��ڹ��˺����Һ�м�������������Һ���Գ�ȥɫ�أ��ٴι��˺�õ���Һ��������Һϡ����1 L��ȡ25.00 mLϡ�ͺ����Һ�������ϡ����͵⻯����Һ��Ӧ����ѡ�ú��ʵ�ָʾ������0.05000 mol��L��1Na2S2O3����Һ���еζ���ʵ�����ݼ�¼���£�
|
����� |
0.050 00 mol��L��1 Na2S2O3����Һ���/mL |
|
|
�ζ�ǰ�̶� |
�ζ���̶� |
|
|
1 |
0.00 |
20.04 |
|
2 |
0.12 |
20.08 |
|
3 |
0.05 |
20.05 |
��ش��������⣺
(1)��ʵ���п�ѡ�õ�ָʾ����________���ζ��յ��������________��
(2)�о���ij�ʦ�����ݲ�ʱ�������������ij�֭���Լ����������ε�Σ��������Ҫ����Ϊ��֭�к��зḻ��ά����C��ά����C����________��(���������ԭ��)��
(3)ͨ�������ж���ij��һ��ʳ��0.125 kg�����ݲˣ��Ƿ�������ж���
һ������£�������һ������ȡ300 mg��500 mg��������ʱ���ͻ������ж���ij�о�С���õ������ⶨ�ݲ����������εĺ������йط�Ӧ���£�
2NaNO2��2H2SO4��2KI===2NO����I2��K2SO4��Na2SO4��2H2O
2Na2S2O3��I2===Na2S4O6��2NaI
ȡ1 kg�ݲˣ�ե֭����ե����Һ���ռ�������ȡ�����������ƣ�ʹ�ݲ�֭�е��������ζ���Ϊ�������ƣ��ڹ��˺����Һ�м�������������Һ���Գ�ȥɫ�أ��ٴι��˺�õ���Һ��������Һϡ����1 L��ȡ25.00 mLϡ�ͺ����Һ�������ϡ����͵⻯����Һ��Ӧ����ѡ�ú��ʵ�ָʾ������0.05000 mol��L��1Na2S2O3����Һ���еζ���ʵ�����ݼ�¼���£�
|
����� |
0.050 00 mol��L��1 Na2S2O3����Һ���/mL |
|
|
�ζ�ǰ�̶� |
�ζ���̶� |
|
|
1 |
0.00 |
20.04 |
|
2 |
0.12 |
20.08 |
|
3 |
0.05 |
20.05 |
��ش��������⣺
(1)��ʵ���п�ѡ�õ�ָʾ����________���ζ��յ��������________��
(2)�о���ij�ʦ�����ݲ�ʱ�������������ij�֭���Լ����������ε�Σ��������Ҫ����Ϊ��֭�к��зḻ��ά����C��ά����C����________��(���������ԭ��)��
(3)ͨ�������ж���ij��һ��ʳ��0.125 kg�����ݲˣ��Ƿ�������ж���
________________________________________________________________________
________________________________________________________________________