��Ŀ����
����٤������ԼΪ6.02��1023mol-1������������ȷ����
- A.8.2 g Na218O2�������H216O���ã����ɵ������к��е�������Ϊ0.8��6.02��1023
- B.6.4g CaC2�����к���������������Ϊ0.3��6.02��1023
- C.0.88g C3H8�к��еĹ��ۼ�����Ϊ0.2��6.02��1023
- D.7.8g �������Ʒ�ĩ��ˮ��Ӧת�Ƶĵ�����Ϊ0.2��6.02��1023
C
������A��������Է�����������������Ƶ����ʵ���������ԭ����ɼ�����������
B��������Է�����������̼���Ƶ����ʵ�����̼�������������Ǹ����ӣ���������C22-���ٽ�Ͼ���Ĺ��������������Ӹ�����
C��������Է������������������ʵ�����һ����������к���10���ۼ������ݱ���Ĺ��ɼ��㹲�ۼ�������
D�����ݹ������ƺ�ת�Ƶ���֮��Ĺ�ϵ����ת�Ƶ���������
���A.8.2g Na218O2�����ʵ���=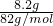 =0.1mol�����ɵ���������ʽ18O2��һ�����������к���20�����ӣ����Ժ��е�������=0.05mol��6.02��1023��20=6.02��1023����A����
=0.1mol�����ɵ���������ʽ18O2��һ�����������к���20�����ӣ����Ժ��е�������=0.05mol��6.02��1023��20=6.02��1023����A����
B.6.4g CaC2�����ʵ���= =0.1mol��һ��̼���ƻ�ѧʽ�к���1��������1�������ӣ�����������������=0.2��6.02��1023����B����
=0.1mol��һ��̼���ƻ�ѧʽ�к���1��������1�������ӣ�����������������=0.2��6.02��1023����B����
C��һ����������к���10���ۼ���0.88g C3H8�����ʵ���=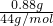 =0.02mol�����Ժ��еĹ��ۼ�����=0.2��6.02��1023����C��ȷ��
=0.02mol�����Ժ��еĹ��ۼ�����=0.2��6.02��1023����C��ȷ��
D.78g �������Ʒ�ĩ��ˮ��Ӧת�Ƶĵ�����Ϊ6.02��1023����7.8g�������Ʒ�ĩ��ˮ��Ӧת�Ƶĵ�����Ϊ0.1��6.02��1023����D����
��ѡC��
���������⿼�鰢���ӵ���������Ŀ�ѶȲ��������ʵ���ɡ��ṹ��������������ɣ�ע��̼���ƵĻ�ѧʽ�к���һ��������һ�������ӣ�Ϊ�״��㣮
������A��������Է�����������������Ƶ����ʵ���������ԭ����ɼ�����������
B��������Է�����������̼���Ƶ����ʵ�����̼�������������Ǹ����ӣ���������C22-���ٽ�Ͼ���Ĺ��������������Ӹ�����
C��������Է������������������ʵ�����һ����������к���10���ۼ������ݱ���Ĺ��ɼ��㹲�ۼ�������
D�����ݹ������ƺ�ת�Ƶ���֮��Ĺ�ϵ����ת�Ƶ���������
���A.8.2g Na218O2�����ʵ���=
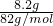 =0.1mol�����ɵ���������ʽ18O2��һ�����������к���20�����ӣ����Ժ��е�������=0.05mol��6.02��1023��20=6.02��1023����A����
=0.1mol�����ɵ���������ʽ18O2��һ�����������к���20�����ӣ����Ժ��е�������=0.05mol��6.02��1023��20=6.02��1023����A����B.6.4g CaC2�����ʵ���=
 =0.1mol��һ��̼���ƻ�ѧʽ�к���1��������1�������ӣ�����������������=0.2��6.02��1023����B����
=0.1mol��һ��̼���ƻ�ѧʽ�к���1��������1�������ӣ�����������������=0.2��6.02��1023����B����C��һ����������к���10���ۼ���0.88g C3H8�����ʵ���=
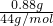 =0.02mol�����Ժ��еĹ��ۼ�����=0.2��6.02��1023����C��ȷ��
=0.02mol�����Ժ��еĹ��ۼ�����=0.2��6.02��1023����C��ȷ��D.78g �������Ʒ�ĩ��ˮ��Ӧת�Ƶĵ�����Ϊ6.02��1023����7.8g�������Ʒ�ĩ��ˮ��Ӧת�Ƶĵ�����Ϊ0.1��6.02��1023����D����
��ѡC��
���������⿼�鰢���ӵ���������Ŀ�ѶȲ��������ʵ���ɡ��ṹ��������������ɣ�ע��̼���ƵĻ�ѧʽ�к���һ��������һ�������ӣ�Ϊ�״��㣮

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ
����٤������ԼΪ6.02��1023mol-1������������ȷ���ǣ�������
| A��25��ʱ��1L pH=13��NaOH��Һ��Լ����6.02��1023������������ | B�������£�42.0g��ϩ�ͱ�ϡ�Ļ�������к��е�̼ԭ����ԼΪ3��6.02��1023 | C����״���£�2.24L����Լ����3.612��1023��̼ԭ�� | D��1.0L 1.0mol/L CH3COOH��Һ��CH3COOH������Ϊ6.02��1023 |