��Ŀ����
��1����ʵ��������С��ʵ�飺������A��B��һ����������һ�������Ϊ300cm2��Բ��������ʹѹǿΪp��Ȼ��������ϵ�ü��������ȵ�280��ʱ���������·�Ӧ��
3A(g)+B(g)![]() 2C(g)+2D(g)+180kJ
2C(g)+2D(g)+180kJ
��ƽ��ÿ��������0.5mol��C��������������ɢ������ƽ��Ϊ0.4kJ��min-1��cm-2��Ϊ��ά�ֺ���280�棬ƽ��ÿ�������ü���������ϵ�ṩ����ǧ���������������������̣�______________��
��2��������1����С��ʵ������Ϊ��ҵ����������Ӧ�����ĵ���뾶�߶ȶ�����ԭ������10�������ɡ������塱���������н�A��B��ԭ�������룬ʹѹǿ��Ϊp��Ȼ����ȵ�280��ʹ��Ӧ��ʼ��Ϊ��ά�ֺ���280�棬��Ӧ��ʼ��Ӧ�������Ȼ��ǽ�����ȴ?������������ɢ�����ʲ��䣩ƽ��ÿ�����ü���������ϵ�ṩ��������ȴ�����ն���ǧ���������������������̣�______________��
��3���ں��¡���ѹ�£���2���ġ������塱��ƽ����ϵ�У��ٽ�A��B��ԭ�������룬���´ﵽƽ��ʱ��C���������_______��
������
��1��75 ��2��33000 ��3������
|
��ʾ��
��ʾ����1����ά�ֺ���280�棬��������ϵΪ���������ṩ����+��Ӧ�ų�����=��������ɢ���������� �� ��1min�ƣ�����0.5mol C��������180kJ´ 0.4kJ��Min-1��cm-2´300cm-2´1min=120kJ�����ԣ�Ӧ�ü�����ÿ�����ṩ����120kJ-45KJ=75kJ�� ��2�������뾶�߶����ԭ����10������������Ϊԭ����100�����ݻ���Ϊԭ����1000����ƽ��ÿ��������CΪ0.5mol´1000=500mol���ų�����45000KJ��������ɢ��12000KJ������ȴ��ÿ����Ӧ���յ�45000kJ-12000KJ=3300014�������� ��3�����º�ѹ�£���ƽ����ԭƽ���Ч������C������������䡣
|

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д���һ�����ʵ���Ũ����Һ�����ƺ�����к͵ζ�����ѧ��ѧ���������͵Ķ���ʵ�顣ij�о���ѧϰС����ʵ����������1mol/L��ϡ�������Һ��Ȼ������ζ�ijδ֪Ũ�ȵ�NaOH��Һ�������й�˵������ȷ����______________���𰸿��ܲ�Ψһ��
| A��ʵ�������õ��ĵζ��ܡ�����ƿ����ʹ��ǰ����Ҫ��©�� |
| B�����ʵ��������60mL ��ϡ�������Һ������ʱӦѡ��100mL����ƿ�� |
| C������ƿ�к�����������ˮ���ᵼ���������Һ��Ũ��ƫС�� |
| D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ���õ�NaOH��Һ��Ũ�Ƚ�ƫ�� |
F��������Һ���к͵ζ�������ʵ���У��������һ�ζ��������Ӷ���������ʵ������ƫ��
�� . ������ͼ��ʾ��װ����ȡ�϶����ı�����ˮ���ⶨ������ˮ��pH��
�ش��й����⣺
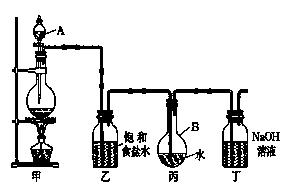
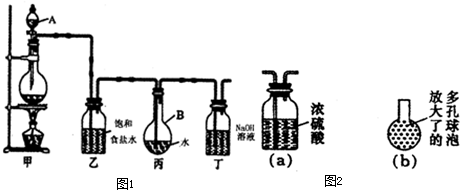
��1��д���йػ�ѧ����ʽ��
װ�üף�____________________________________ ��
װ�ö��� __________________ ��
��2��֤����ˮ�ѱ��͵������� ��
��3����ȡ����ʱ��װ�ñ���Һ���к��е����� ����
 �����ű�ʾ ����
�����ű�ʾ ����(4)����ȥװ���ң�ֱ�ӽ�װ�üͱ���������������ʵ��ⶨ�����Ӱ���ǣ� ��
�ⶨ������ˮ��pH������_______________________________________________��
��.ʵ����ƣ�֤��NaOH�����ڿ����з��ò��ֱ���
_______________________________________________________________
 _______________________________________________________________________
_______________________________________________________________________