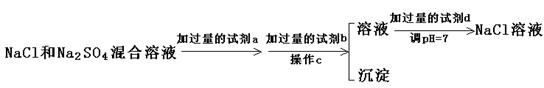
��Ŀ����
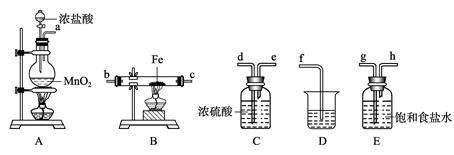
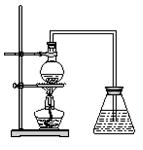
��15�֣�ij����С�齫��ͼ��ʾװ�ð�һ��˳�����ӣ���ʵ��������ȡһ������FeCl3����ͨ�����������ַ�Ӧ����
��ش��������⣺
��1�� A�з�����Ӧ�Ļ�ѧ����ʽΪ______ _________________________________��
_________________________________��
��2����װ�õ���ȷ����˳��Ϊ����дװ�ô��ţ�A��______��______��______��
______D��
��3��װ��C��������________________________________________________��
д��װ��D�з�Ӧ�����ӷ���ʽ___________ _________________________��
_________________________��
��4����Ӧ��ʼ��B��Ӳ�ʲ������ڵ�����Ϊ______________________________ ��
��
���Լ����������к���Fe3+���Լ���____________����д�Լ����ƣ���
(5) ��С��������ͼ��ʾװ���ռ�β������������������������
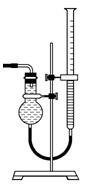
������ ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
��Ϊ��߲�����ȷ�ԣ���ͼװ���е�Һ�����________��
�ռ����������ǰӦ���еIJ�����____________��
�������ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬 �ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
�ᵼ��������������__________���ƫ����ƫС������Ӱ�족����

��ش��������⣺
��1�� A�з�����Ӧ�Ļ�ѧ����ʽΪ______
 _________________________________��
_________________________________����2����װ�õ���ȷ����˳��Ϊ����дװ�ô��ţ�A��______��______��______��
______D��
��3��װ��C��������________________________________________________��
д��װ��D�з�Ӧ�����ӷ���ʽ___________
 _________________________��
_________________________����4����Ӧ��ʼ��B��Ӳ�ʲ������ڵ�����Ϊ______________________________
 ��
�����Լ����������к���Fe3+���Լ���____________����д�Լ����ƣ���
(5) ��С��������ͼ��ʾװ���ռ�β������������������������

������
 ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ���Ϊ��߲�����ȷ�ԣ���ͼװ���е�Һ�����________��
�ռ����������ǰӦ���еIJ�����____________��
�������ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬
 �ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
�ᵼ��������������__________���ƫ����ƫС������Ӱ�족������15�֣���1�� 4HCl(Ũ)+MnO2 MnCl2+Cl2��+2H2O��2�֣�
MnCl2+Cl2��+2H2O��2�֣�
��2�� E��C��B��2�֣�
��3������Cl2����ֹFeCl3��ˮ�⣨1�֣���Cl2+2OH�C = Cl�C + ClO�C+H2O��2�֣�
��4���أ��죩ɫ���̣�1�֣��������� �أ�1�֣�
�أ�1�֣�
��5���ټ�ʽ��1�֣�
�ڱ���NaCl��Һ��1�֣��������ƶ��ζ��ܣ�ʹ��������Һ����ƽ��2�֣�
��ƫ��2�֣�
 MnCl2+Cl2��+2H2O��2�֣�
MnCl2+Cl2��+2H2O��2�֣���2�� E��C��B��2�֣�
��3������Cl2����ֹFeCl3��ˮ�⣨1�֣���Cl2+2OH�C = Cl�C + ClO�C+H2O��2�֣�
��4���أ��죩ɫ���̣�1�֣���������
 �أ�1�֣�
�أ�1�֣�
��5���ټ�ʽ��1�֣�
�ڱ���NaCl��Һ��1�֣��������ƶ��ζ��ܣ�ʹ��������Һ����ƽ��2�֣�
��ƫ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��
��
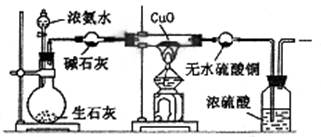
 2CuO,CuO+2HNO3="=" Cu(NO3)2 + H2O
2CuO,CuO+2HNO3="=" Cu(NO3)2 + H2O