��Ŀ����
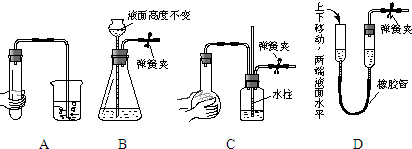
��ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش���1��ָ���������������ƣ�
A______��
D______��
��2����A��ΪŨ��ˮ��B��Ϊ�ռC��ΪAICl3��Һ��ʵ���пɹ۲B�����������ɣ�C���а�ɫ��������C�з�����Ӧ�����ӷ���ʽΪ______������D������Ϊ______��
��3����A��װ��Ũ���ᣬB��װ�й���KMn04��C��ʢ��KI������Һ��ʵ���пɹ۲B�г��ֻ���ɫ���壬C����Һ����������ʵ��������Եõ��Ľ���Ϊ��______��
��������ǿ������˳��______��
����װ�õIJ���֮���ǣ�______��
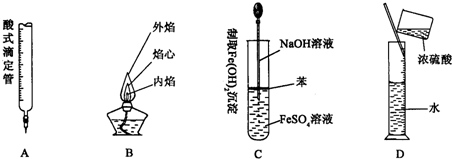
���𰸡���������1������ͼ����ѧ���������Ľṹ�����
��2���ռ��백ˮ��ϣ���Һ��������Ũ�����ų��������ȣ����백���ӷ����ӷ��İ�����C��������ˮ���Ȼ�����Ӧ��������������ɫ����������װ����ѹǿ���ͣ�����D�ɷ�ֹ������
��3��B�г��ֻ���ɫ���壬˵��Ũ������KMn04��Ӧ����������C����Һ������˵��������C���������������ɵⵥ�ʣ�������������������ǿ����������������ԣ��ж����ʵ������ԣ�
�����ж������ܽ�β��ֱ���ŷŵ������У�
����⣺��1����ͼ��֪AΪ��Һ©����DΪ����ܣ�
�ʴ�Ϊ����Һ©���� ����ܣ�
��2���ӷ��İ�����C��������ˮ���Ȼ�����Ӧ��������������ɫ��������Ӧ���ӷ���ʽΪ
Al3++3NH3?H2O�TAl��OH��3+3NH4+��
������Ӧ����װ����ѹǿ���ͣ�������Һ����������D�ɷ�ֹ������
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3+3NH4+����ֹ������
��3��B�г��ֻ���ɫ���壬˵��Ũ������KMn04��Ӧ����������������C����Һ������˵��������C���������������ɵⵥ�ʣ����Կɵ�������ǿ��˳��ΪKMnO4��Cl2��I2�������ж������ܽ�β��ֱ���ŷŵ������У�ʵ��ȱ��β������װ�ã�
�ʴ�Ϊ��KMnO4��Cl2��I2��ȱ��β������װ�ã�
������������ʵ��Ϊ���壬�����������ʶ��Ԫ�ػ�����֪ʶ�������ԱȽϡ���ʵ��װ�õ����۵ȣ���Ŀ�ѶȲ���ע�����֪ʶ���գ�
��2���ռ��백ˮ��ϣ���Һ��������Ũ�����ų��������ȣ����백���ӷ����ӷ��İ�����C��������ˮ���Ȼ�����Ӧ��������������ɫ����������װ����ѹǿ���ͣ�����D�ɷ�ֹ������
��3��B�г��ֻ���ɫ���壬˵��Ũ������KMn04��Ӧ����������C����Һ������˵��������C���������������ɵⵥ�ʣ�������������������ǿ����������������ԣ��ж����ʵ������ԣ�
�����ж������ܽ�β��ֱ���ŷŵ������У�
����⣺��1����ͼ��֪AΪ��Һ©����DΪ����ܣ�
�ʴ�Ϊ����Һ©���� ����ܣ�
��2���ӷ��İ�����C��������ˮ���Ȼ�����Ӧ��������������ɫ��������Ӧ���ӷ���ʽΪ
Al3++3NH3?H2O�TAl��OH��3+3NH4+��
������Ӧ����װ����ѹǿ���ͣ�������Һ����������D�ɷ�ֹ������
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3+3NH4+����ֹ������
��3��B�г��ֻ���ɫ���壬˵��Ũ������KMn04��Ӧ����������������C����Һ������˵��������C���������������ɵⵥ�ʣ����Կɵ�������ǿ��˳��ΪKMnO4��Cl2��I2�������ж������ܽ�β��ֱ���ŷŵ������У�ʵ��ȱ��β������װ�ã�
�ʴ�Ϊ��KMnO4��Cl2��I2��ȱ��β������װ�ã�
������������ʵ��Ϊ���壬�����������ʶ��Ԫ�ػ�����֪ʶ�������ԱȽϡ���ʵ��װ�õ����۵ȣ���Ŀ�ѶȲ���ע�����֪ʶ���գ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


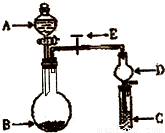
 ��2012?��ɫ��ģ��ij��ѧ��ȤС���ͬѧ����֤Mg��CO2�ķ�Ӧ������ѡ�õ�ҩƷ��Ũ���ᡢϡ���ᡢϡ���ᡢþ�ۡ�����ʯ������ʯ��ˮ������NaHCO3��Һ������Na2CO3��Һ����ѡ�õ�װ�ã�����ͼ��ʾ����Ҫʱװ�ÿ��ظ�ʹ�ã��˴����ӳ�һ��������ʵ��װ�ã�
��2012?��ɫ��ģ��ij��ѧ��ȤС���ͬѧ����֤Mg��CO2�ķ�Ӧ������ѡ�õ�ҩƷ��Ũ���ᡢϡ���ᡢϡ���ᡢþ�ۡ�����ʯ������ʯ��ˮ������NaHCO3��Һ������Na2CO3��Һ����ѡ�õ�װ�ã�����ͼ��ʾ����Ҫʱװ�ÿ��ظ�ʹ�ã��˴����ӳ�һ��������ʵ��װ�ã�