��Ŀ����
(1)����������ѧ��ѧʵ���г��õ�����������ʵ������У�һ�㲻��Ҫ�ò���������________(��д���)��
����pH��ֽ�ⶨNa2CO3��Һ��pH
������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ
�۽������Ȼ���������Һ�����ˮ���Ʊ�������������
��̽��Ba(OH)2��8H2O�����NH4Cl���巴Ӧ�����е������仯
��ʵ���������Ƶ�FeSO4��Һ��Ԥ��������NaOH��Һ�Ʊ�Fe(OH)2��ɫ����
(2)�á����ڡ�����С�ڡ����ڡ���գ�
�ٶ�ȡ��Ͳ��Һ������ʱ������ƫ�ߣ���ȡ�������________ʵ���������
����������ƽ��ȡ10.4 gʳ�Σ��������ʳ�ε�λ�õߵ�������ȡʳ�ε�����_____10.4 g��
������500 mL 0.1 mol/L NaOH��Һ������ʱ���ӿ̶��ߣ�������Һ�����ʵ���Ũ�� _______0.1 mol/L��
�����к͵ζ�����ijNaOH��Һ��Ũ�ȣ���ȡ����Һʱδ�ø���Һ��ϴ�ζ��ܣ���õ���ҺŨ��________ʵ��Ũ�ȡ�
(1) �ۢ� (2)�ٴ��� ��С�� �۴��� ��С��
��������
�����������1���������ǻ�ѧʵ���г��õ������������������ڽ��衢���˻�ת��Һ��ʱ���������ݸ�ʵ���ʵ��Ŀ��ȷ���Ƿ���Ҫ��������
��2���ٸ�����Ͳ�Ķ������µ�����ֵ���������ݸ���ʱ�Ķ�����Һ��Ĺ�ϵ���з�����
�ڸ�����ƽ�ij���ԭ��������=����+����Ķ�����������
�۸��ݸ���ʱ�Ŀ̶�����Һ��Ĺ�ϵ���з�������Ĵ�С���ٸ���c��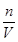 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ����nƫ���VƫС������������ҺŨ��ƫ�ߣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ����nƫ���VƫС������������ҺŨ��ƫ�ߣ�
�ܸ�����ȡ����Һʱĩ�ø���Һ��ϴ�ζ��ܣ�����ҺʱŨ�Ƚ���С��һ���������Һ�����ʵ�������С�����ı�Һ�����ƫС������Һ��Ũ��ƫС��
��1������pH��ֽ�ⶨNa2CO3��Һ��pH���ò�����պȡ��������Һ����ֽ���в����ʢٴ���
������һ�����ʵ���Ũ�ȵ��Ȼ�����Һʱ���ܽ���Ҫ���������裬ת����Ҫ�������������ʢڴ���
�۽������Ȼ���������Һ�����ˮ���Ʊ������������壬����ֱ��Һ���Ϊ���ɫֹͣ���ȣ����貣�������裬�ʢ���ȷ��
��̽��Ba��OH��2•8H2O�����NH4Cl���巴Ӧ�����е������仯���ò���������������Ļ����ʢܴ���
��ʵ���������Ƶ�FeSO4��Һ��Ԥ��������NaOH��Һ�Ʊ�Fe��OH��2��ɫ������������NaOH��Һ�ĵιܲ���Һ�����£����貣�������ʢ���ȷ����ѡ�ۢݡ�
��2��������Ͳ�Ķ������µ�����ֵ��������ʱ�Ķ�����Һ��ߣ����Զ�ȡ�����������ʵ���������
������ƽ�ij���ԭ�������̣�����+����Ķ�������������ƽ��ȡ10.4gʳ�Σ��������ʳ�ε�λ�õߵ����Ƶõ�����Ϊ9.6gʳ�Σ�
������ʱ�Ŀ̶��߱�Һ��ߣ���Һ�����ƫС��Ũ��ƫ��
������ȡ����Һʱĩ�ø���Һ��ϴ�ζ��ܣ����Դ���ҺʱŨ�Ƚ���С��һ���������Һ�����ʵ�������С�����ĵı�Һ���ƫС����õ���ҺŨ��С��ʵ��Ũ�ȡ�
���㣺���黯ѧʵ�����������������
����������ʱ�߿��еij������ͣ������е��Ѷȵ����⣬���������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺ͼ��飬����������ѧ���淶�Ͻ���ʵ����������Ͷ��ֲ�����������ѧʵ�����������ƽʱ��ѧϰ��ע����ۺ��ܽᣬ����������á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ����С�ձ��ڼ����������ữ��ϡKI������Һ����С�ձ����ڱ����·���
����С�ձ��ڼ����������ữ��ϡKI������Һ����С�ձ����ڱ����·���