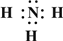��Ŀ����
X��Y��Z��L��M��N����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�Ƕ�����ԭ�Ӱ뾶����Ԫ�أ�N�ǵؿ��к�����ߵĽ���Ԫ�ء�
�û�ѧ����ش��������⣺
��1�� M��Ԫ�����ڱ��е�λ��Ϊ ������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3���ɷ���A, A�ĵ���ʽΪ ��Y��L��Ԫ�ذ�ԭ����Ŀ��l��2���ɷ���B��B�������Ļ�ѧ������Ϊ ��
��3������se��������������Ԫ�أ���֪�ǽ����ԣ�34Se<L������ԭ�ӽṹ����ԭ�� ��
��4����Y��L��M���ɵ�������Һ����������ۣ��������ӷ���ʽ�Լ���Ҫ�����ֽ���ԭ�� ��
��5����ʯī���缫��NCl3��Һ�����Һ���е�⣬����������R��R���ȷֽ����ɻ�����Q��д���������Q��ȡN�ĵ缫����ʽ�������� �������� ��
��1���������ڡ��ڢ�A�壨1�֣���Na>Al>C>N>O>H��2�֣�
��2�� ��1�֣������Թ��ۼ���1�֣�
��1�֣������Թ��ۼ���1�֣�
��3��O��Seͬ�壬ԭ�Ӱ뾶���������µõ����������������Էǽ����Լ�������2�֣�
��4�� CO32- + H2O 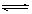 HCO3-
+OH-��̼������Һˮ���Լ��ԣ���ʹ��֬�ڼ���������ˮ��Ӷ��ܽ⡣��2�֣�
HCO3-
+OH-��̼������Һˮ���Լ��ԣ���ʹ��֬�ڼ���������ˮ��Ӷ��ܽ⡣��2�֣�
��5��2O2--4e-=O2����2�֣���Al3+ +3e-=Al ��2�֣�
��������
�����������1��ԭ�Ӱ뾶��Ҫ�ɵ��Ӳ������˵����������ͬ����Ԫ�ص��Ӳ�����ͬ��ԭ�Ӱ뾶�ɺ˵�����������˵����ԽС���뾶Խ������M������ԭ�Ӱ뾶����Ԫ����Na��λ��Ԫ�����ڱ��ĵ������ڵ�һ���壻���ɵ����ʵĻ���Ԫ��X��Y��Z��L�ֱ���H��C��N��O���ؿ��к������Ľ���Ԫ����Al������N��AlԪ�أ�����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳��ΪNa>Al>C>N>O>H
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3���ɷ���A,A��NH3�������ʽΪ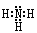 Y��L��Ԫ�ذ�ԭ����Ŀ��l��2���ɷ���B��BΪCO2��CO2�������Ļ�ѧ������Ϊ���Թ��ۼ���
Y��L��Ԫ�ذ�ԭ����Ŀ��l��2���ɷ���B��BΪCO2��CO2�������Ļ�ѧ������Ϊ���Թ��ۼ���
��3��O��Se��ͬ����Ԫ�أ����ϵ�����ԭ�Ӱ뾶������Ԫ��ԭ�ӵõ��ӵ��������������Էǽ����Լ���
��4����Y��L��M���ɵ�������ҺΪ̼������Һ��CO32-ˮ��CO32- + H2O 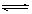 HCO3-
+OH-��ʹ��Һ�Լ��ԣ���ʹ��֬�ڼ���������ˮ��Ӷ��ܽ�
HCO3-
+OH-��ʹ��Һ�Լ��ԣ���ʹ��֬�ڼ���������ˮ��Ӷ��ܽ�
��5����ʯī���缫�����AlCl3��Һ,�͵��ʳ��ˮ���ƣ��õ�Al(OH)3��Al(OH)3���ȷֽ����ɻ�����QAl2O3,�������Al2O3ʱ��������O2-�ŵ磬�����������缫��Ӧ����ʽ��2O2--4e-=O2����������Al3+�ŵ磬�缫����ʽΪAl3+ +3e-=Al
���㣺����ԭ�ӽṹ��Ԫ�����ʵĹ�ϵ��ԭ�Ӱ뾶�ıȽϣ��ǽ����ԵıȽϣ��ε�ˮ�⣬��ⷨ����

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�