��Ŀ����
A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������A��C��B��D�ֱ���ͬ����Ԫ�أ���֪B��D��Ԫ��ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵�2��������Ԫ���γɵĵ����������������壻�����ǹ��塣��ش��������⣺
��1��д����A��B��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����Ϊ__________���˻�������ʹ����KMnO4��Һ��ɫ�����ָû�������� �ԡ�
��2��D������������Ӧˮ������һ����Ҫ�Ĺ�ҵ��Ʒ�����й��ڴ˻�����������ȷ���ǣ� ��
A����Ũ��Һ����ǿ�����ԣ����������������ʷ�Ӧ
B����ϡ��Һ������ˮ�ԣ��ܾ�����������
C�������ʾ�����ˮ�ԣ�����ϩ��ʵ�����Ʊ�����ֱ������
 D�������ʿ�������������������
D�������ʿ�������������������
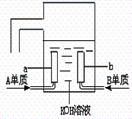
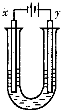
��3����AԪ�صĵ�����BԪ�صĵ��ʿ��Ƴ����͵Ļ�ѧ��Դ����������ɴ���ʹ�á��乹������ͼ��ʾ�������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ硣
��a�� �����缫��ӦʽΪ____________________��
b�� �����缫��ӦʽΪ____________________��
��1��H2O2����1�֣���ԭ��1�֣���2��B ��2�֣�
��3��������1�֣���H2+2OH-�D2e-=2H2O��2�֣�
������1�֣���O2+2H2O+4e -=4 OH-��2�֣�

 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д� ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ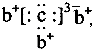 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
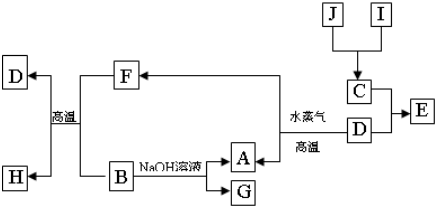
 Al��OH��3+OH-
Al��OH��3+OH-