��Ŀ����
������Һ���й����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ� ��
A����0.2 mol/L��ijһԪ����HA��Һ��0.1 mol/LNaOH��Һ�������ϣ����ַ�Ӧ��Ļ�
��Һ�У�2c(OH��)��c(A��)��2c(H��)��c(HA)
B��pH��ȵ�CH3COONa��Һ��C6H5ONa��Һ��NaHCO3��Һ��NaOH��Һ�������ʵ���Ũ����
С�����˳��Ϊ��c (NaOH)��c(CH3COONa)��c (NaHCO3)��c (C6H5ONa)
C�������£���pH��2.0��CH3COOH��Һ��pH��12.0��NaOH��Һ�������ϣ����ַ�Ӧ
��Ļ��Һ�У�c (Na��)��c (CH3COO��)��c(OH��)��c (H��)
D�������£���10mL pH��4.0�Ĵ�����Һ�м���ˮϡ�ͺ���Һ��c (H��)��c(OH��)��
c (CH3COOH)��c (CH3COO��)����Ҫ��С
A
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��NAΪ����٤����������ֵ������˵����ȷ����
| A�������£�23g NO2����NA����ԭ�� |
| B����״���£�11.2L SO3�к��еķ�����Ϊ0.5NA |
| C��1 mol Cl2��������ת�Ƶĵ�������NA |
| D��1L 0.1mol/L-1�İ�ˮ�к�OH�D������Ϊ0.1NA�� |
���й���ͬ��ͬѹ�µ���������12C18O��14N2���ж���ȷ����(����)
| A���ܶ�֮��Ϊ14:15 | B��������ʱ���еĵ�������� |
| C��ԭ�������ʱ���е���������� | D���������ʱ���е���������� |
�����йػ�ѧ����ʹ����ȷ����
A��CO2�ĵ���ʽ��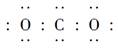 | B��������Ϊ20����ԭ�ӣ� |
C����ϩ�ı���ģ�ͣ�  | D�������ӽṹʾ��ͼ�� |
��Ϊ�����ӵ�������ֵ������˵����ȷ����
A�����³�ѹ�£�15g��(��CH3)������������Ϊ6 |
B��һ��������������Fe����Ũ���ᷴӦ��ת�Ƶ�����һ��Ϊ3 |
C����1L��̼������Һ�У���c(CO32��)=1mol/L����Na������Ϊ2 |
D����4 �����ӵĹ���Na2O2����ˮ���1L��Һ��������Һ��Na����Ũ��Ϊ1mol/L �����ӵĹ���Na2O2����ˮ���1L��Һ��������Һ��Na����Ũ��Ϊ1mol/L |
�����йػ�ѧ�����ʹ����ȷ����
A��HCl�ĵ���ʽ�� |
B��þ��ԭ�ӽṹʾ��ͼ�� |
C���廯�Ƶĵ���ʽ��  |
| D��Na2SO4�ĵ��뷽��ʽ��Na2SO4��Na++ SO42- |
��NA��ʾ�����ӵ�������ֵ������˵����ȷ����
| A����״���£�0. 1 mol Cl2����ˮ��ת�Ƶĵ�����ĿΪ0. 1NA |
| B�����³�ѹ�£�18 g H2O �к��е�ԭ������Ϊ3NA |
| C����״���£�11. 2 L CH3CH2OH �к��еķ�����ĿΪ0. 5NA |
| D�����³�ѹ�£�2. 24 L CO ��CO2��������к��е�̼ԭ����ĿΪ0. 1NA |
���л�ѧ�����ʾ��ȷ����
| A����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��2H2��g��+O2��g��= 2H2O��1����H= -571.6kJ/mol |
B����������Ҫ�ɷ�ΪRCOONa����ˮ��Һ�Լ��ԣ�RCOO-+H2O RCOOH+OH- RCOOH+OH- |
C��ʵ���������������ӷ���ʽ��MnO2+4H++4Cl�� MnCl2+2H2O+Cl2�� MnCl2+2H2O+Cl2�� |
D���������Ҵ���Ӧ�Ļ�ѧ����ʽ��CH3CO18OH+C2H5OH CH3CO18OC2H5+H2O CH3CO18OC2H5+H2O |
