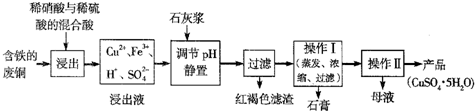
��Ŀ����
CuSO4��5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�������CuSO4��5H2O��ʵ�����Ʊ�����ͼ��
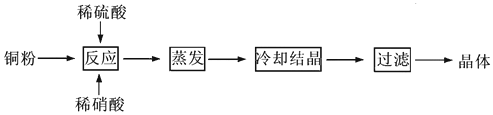
�����������������գ�
��1�������ϣ�Ϊ���Ƶô�����CuSO4��5H2O���壬��Ҫ����ϡ���ᡢϡ�����������ʵ���֮��Ϊ__________��������Ӧ�����ӷ���ʽΪ____________________��
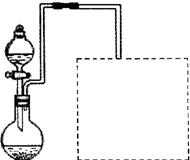
��2��Ϊ�ӿ�����ٶȣ��ɲ���__________���������ƣ����ò�����ϴ�ӳ���ʱ����Ҫע��ϴ�Ӽ���ѡ�����������Ӧ____________________
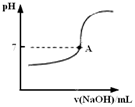
��3��ʵ�����������ж�����ϡ�����Ũ�ȿ���Ҫ��Ƚϸߣ�ͨ���ñ�����������Һ���ζ����ζ����������÷�̪��ָʾ�����յ�������____________________��������ͼ�л����ζ���������Һ��pH�����μ�����������Һ����ı仯������ͼ��Ҫ���A�㣩��
��1�������ϣ�Ϊ���Ƶô�����CuSO4��5H2O���壬��Ҫ����ϡ���ᡢϡ�����������ʵ���֮��Ϊ__________��������Ӧ�����ӷ���ʽΪ____________________��
��2��Ϊ�ӿ�����ٶȣ��ɲ���__________���������ƣ����ò�����ϴ�ӳ���ʱ����Ҫע��ϴ�Ӽ���ѡ�����������Ӧ____________________
��3��ʵ�����������ж�����ϡ�����Ũ�ȿ���Ҫ��Ƚϸߣ�ͨ���ñ�����������Һ���ζ����ζ����������÷�̪��ָʾ�����յ�������____________________��������ͼ�л����ζ���������Һ��pH�����μ�����������Һ����ı仯������ͼ��Ҫ���A�㣩��
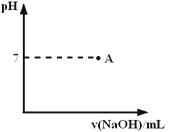
��4������ʹ�õı�����������Һ�Ѿ�ͨ�������ʵı궨������������ͨ���������궨��Һ�Ļ�������__________ ��
A������ B������ C�������� D������
��5���ö��Ե缫���һ��Ũ�ȵ�����ͭ��Һ��ͨ��һ��ʱ��������õ���Һ�м���0.1mol Cu2(OH)2CO3��ǡ�ûָ������ǰ��Ũ�Ⱥ�pH(������CO2���ܽ�)����������й�ת�Ƶ��ӵ����ʵ���Ϊ__________��
A������ B������ C�������� D������
��5���ö��Ե缫���һ��Ũ�ȵ�����ͭ��Һ��ͨ��һ��ʱ��������õ���Һ�м���0.1mol Cu2(OH)2CO3��ǡ�ûָ������ǰ��Ũ�Ⱥ�pH(������CO2���ܽ�)����������й�ת�Ƶ��ӵ����ʵ���Ϊ__________��
��1��3:2��3Cu +8H+ +2NO3- = 3Cu2+ + 2NO�� +4H2O
��2����ѹ���ˣ���С�����õ�ˮ��ͷ����ϴ�Ӽ�����ͨ��������
(3) ��Һ����ɫ��ɺ�ɫ����dz��ɫ�����Ұ�����ڲ���ɫ
��2����ѹ���ˣ���С�����õ�ˮ��ͷ����ϴ�Ӽ�����ͨ��������
(3) ��Һ����ɫ��ɺ�ɫ����dz��ɫ�����Ұ�����ڲ���ɫ
��4��BC
��5��0.6mol
��5��0.6mol

��ϰ��ϵ�д�
�����Ŀ
������������ȷ���ǣ�������
| A�����ö����ЧӦ����Fe��OH��3�����FeCl3��Һ | B����Ʒ����Һ�����ֶ�����̼�Ͷ������� | C����CuSO4?5H2O����ƾ��к��е�ˮ | D����ɫ��ӦΪ��ɫ��ij��Һ��һ������Na+ |