��Ŀ����
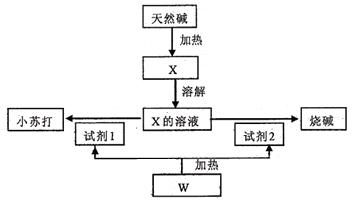
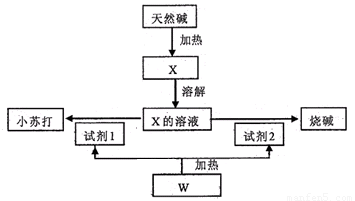
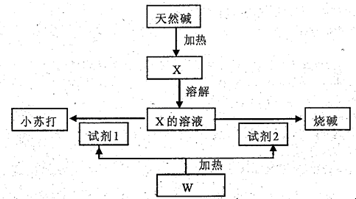
��16�֣�ij��Ȼ��Ļ�ѧ���ΪaNa2CO3��bNaHCO3��cH2O��a��b��cΪ����������������������Ȼ���Ʊ�С�մ���ռ������ͼ��
�ش��������⣺
��1����X����Һ�е����̪��Һ����Һ�� ɫ��ԭ���ǣ�д���ӷ���ʽ��
����������Һ�е��˹�����CaCl2��Һ���۲쵽�������� ��
��2��������X�Ʊ�С�մ���ռ�Ļ�ѧ����ʽ�� ��
��3����ȡ3 32g��Ȼ����Ʒ��ּ����ռ���0.112L�������CO2��0.45g H2O�������ù���������ϡ���������ռ���0.56L�������CO2���壬���������Ȼ��Ļ�ѧ��� ��
��16�֣�
��1���죨2�֣���CO32��+H2OHCO3��+OH�� ��3�֣�
������ɫ����������Һ��ɫ��ȥ ��2�֣�
��2��Na2CO3+CO2+H2O==2NaHCO3 ��3�֣�
Na2CO3+CaO+H2O==2NaOH+CaCO3�� ��3�֣�
�Na2CO3+Ca��OH��2 2NaOH+CaCO3�� ��1��
��3��2Na2CO3��NaHCO3��2H2O ��3�֣�
����:

��ϰ��ϵ�д�
�����Ŀ
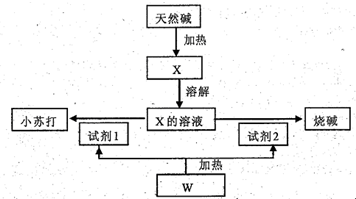
 HCO3-+OH-
HCO3-+OH-