��Ŀ����
S2Cl2�ǹ�ҵ�ϳ��õ�����ʵ�����Ʊ�S2Cl2�ķ�����2�֣�
�� CS2+3Cl2 CCl4+S2Cl2���� 2S+Cl2
CCl4+S2Cl2���� 2S+Cl2 S2Cl2��
S2Cl2��
��֪S2Cl2����Ԫ����+1�ۣ�����ʽ��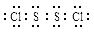 �������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�
�������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�
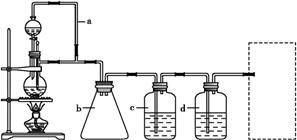
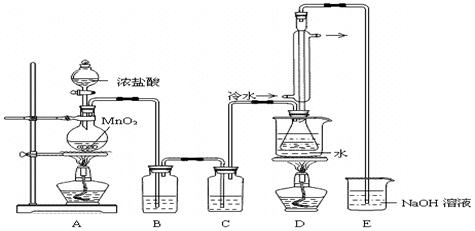
ʵ������������װ���Ʊ�S2Cl2�����ּг���������ȥ����
�ش��������⣺
��1��װ��B��C�в������������ƣ� ����Ӧԭ������д������ţ��� ��
��2��ʵ���������Լ�ͨ������36.5%��Ũ��Һ������ϡ����������� ��
��3��D������������������˫�����ã�����ȴˮ��������������������������ͬ����ͼ����������ȴ��ʽ��Ӧ�������и��л�ѧ�� ʵ�顣
A��ʯ�ͷ��� B����ȡ�屽 C����ȡ�������� D���Ʊ���˾ƥ��
��4��Bװ����ʢ�ŵ��� ����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ����� ��D�в�����ˮԡ���ȵ�ԭ���� ��
��5��A��������װ��ʱ���ź�����̨��Ӧ�ȹ̶� �����������ƣ�������װ��װ����Ϻ�Ӧ�Ƚ��� �������Լ���ʵ����ϣ�A�в��ٲ�������ʱ���ɲ��װ�á����ʱ�����ȵIJ���Ӧ���� ��
��6��ʵ������У���ȱ��Cװ�ã����ֲ�Ʒ���Dz��壬���ָ������ԭ����û�ѧ����ʽ��ʾΪ ��ʵ����ϣ�����ʣ��Ũ���ᵹ��E�ձ�����������β��������������Һ���ʱ����������������ɫ�̼�����������������������ԭ���ǣ� �������ӷ���ʽ��ʾ����
�� CS2+3Cl2
 CCl4+S2Cl2���� 2S+Cl2
CCl4+S2Cl2���� 2S+Cl2 S2Cl2��
S2Cl2����֪S2Cl2����Ԫ����+1�ۣ�����ʽ��
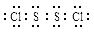 �������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�
�������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�| ���� | S | CS2 | CCl4 | S2Cl2 |
| �е�/�� | 445 | 47 | 77 | 137 |
| �۵�/�� | 113 | -109 | -23 | -77 |
ʵ������������װ���Ʊ�S2Cl2�����ּг���������ȥ����

�ش��������⣺
��1��װ��B��C�в������������ƣ� ����Ӧԭ������д������ţ��� ��
��2��ʵ���������Լ�ͨ������36.5%��Ũ��Һ������ϡ����������� ��
��3��D������������������˫�����ã�����ȴˮ��������������������������ͬ����ͼ����������ȴ��ʽ��Ӧ�������и��л�ѧ�� ʵ�顣
A��ʯ�ͷ��� B����ȡ�屽 C����ȡ�������� D���Ʊ���˾ƥ��
��4��Bװ����ʢ�ŵ��� ����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ����� ��D�в�����ˮԡ���ȵ�ԭ���� ��
��5��A��������װ��ʱ���ź�����̨��Ӧ�ȹ̶� �����������ƣ�������װ��װ����Ϻ�Ӧ�Ƚ��� �������Լ���ʵ����ϣ�A�в��ٲ�������ʱ���ɲ��װ�á����ʱ�����ȵIJ���Ӧ���� ��
��6��ʵ������У���ȱ��Cװ�ã����ֲ�Ʒ���Dz��壬���ָ������ԭ����û�ѧ����ʽ��ʾΪ ��ʵ����ϣ�����ʣ��Ũ���ᵹ��E�ձ�����������β��������������Һ���ʱ����������������ɫ�̼�����������������������ԭ���ǣ� �������ӷ���ʽ��ʾ����
��14�֣�
��1�����ƿ �� ��ÿ��1�֣�
��2��ϡ���ỹԭ��������Ӧ���� ��2�֣�ֻ˵����Ӧ���ѻ�Ӧ��û˵����ԭ����
��1�֣�
��3��BD��2�֣�
��4������ʳ��ˮ ���� ʹCS2ƽ���������������S2Cl2������ÿ��1�֣� l ��ÿ��1�֣�
��5���ƾ��� �����Լ�� ��E�г������ƿ�Һ�� ��ÿ��1�֣�
��6��2S2Cl2+2H2O=3S��+SO2��+4HCl�� ClO-+2H++Cl-=Cl2��+H2O (ÿ��1��)
��1�����ƿ �� ��ÿ��1�֣�
��2��ϡ���ỹԭ��������Ӧ���� ��2�֣�ֻ˵����Ӧ���ѻ�Ӧ��û˵����ԭ����
��1�֣�
��3��BD��2�֣�
��4������ʳ��ˮ ���� ʹCS2ƽ���������������S2Cl2������ÿ��1�֣� l ��ÿ��1�֣�
��5���ƾ��� �����Լ�� ��E�г������ƿ�Һ�� ��ÿ��1�֣�
��6��2S2Cl2+2H2O=3S��+SO2��+4HCl�� ClO-+2H++Cl-=Cl2��+H2O (ÿ��1��)
���������
��1����ʵ��װ��ͼ�п��Կ�����AΪΪ��ȡCl2��B��CΪ��������B��HCl��C����ˮ������D��S������������Ӧ����S2Cl2��
��2����ȡ������Ҫʹ��Ũ���ᣬϡ�����Ӧ��
��3����������������������������ѡBD��
��4��B������������HCl������ʹ�ñ���ʳ��ˮ������ƿ����S��S2Cl2�ȣ�����ʹ������ķ������롣
��5������װ���ԭ���Ǵ��µ��ϣ������ҡ�
��6����ȱ�ٸ���װ�ã����ɵ�S2Cl2��һ���ַ���ˮ������S��SO2�����ܿ������ǡ�EΪCl2��NaOH��Ӧ����NaCl��NaClO������Ũ������ַ�Ӧ����Cl2��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
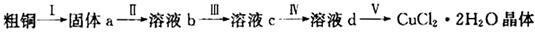

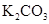
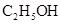 ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������
��Ũ����170�湲�ȣ��Ƶõ�����ͨ������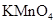 ��Һ
��Һ CH3CH2CH2CHO
CH3CH2CH2CHO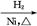 CH3CH2CH2CH2OH;CO���Ʊ�ԭ��:HCOOH
CH3CH2CH2CH2OH;CO���Ʊ�ԭ��:HCOOH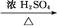 CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��