��Ŀ����
ij��γ�С������50mLNaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�����������NaHCO3�����������ʵ�鲽�裺
a��ȡ25mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b��С�������Һ1��2min��
c���ڵõ�����Һ�м�����һ�루25mL��NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
��1���˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����ʽ______��______��
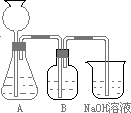
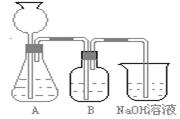
�˷�����һ����ʵ��װ����ͼ��ʾ��
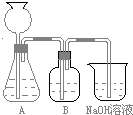
��2�����뷴Ӧ��ǰ����μ������װ�õ������ԣ�______��3��װ��B��ʢ�ŵ��Լ���______��������______��
��4����ʵ����ͨ���Ʒ��У�װ��A������Ϊ����______����ķ���װ�ã�����ţ���
��CH2�TCH2 ��H2S ��CH4 ��CH��CH ��H2
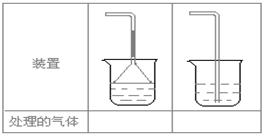
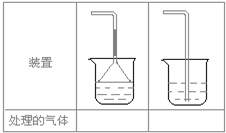
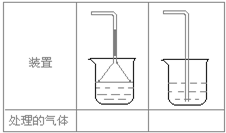
��5��ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4����CO����CO2����O2ʱ�����ڱ������β����������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڣ�
��6����֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸��� Һ�ܶ�Ϊ1.44g/mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ______��
a��ȡ25mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b��С�������Һ1��2min��
c���ڵõ�����Һ�м�����һ�루25mL��NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
��1���˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����ʽ______��______��
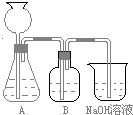
�˷�����һ����ʵ��װ����ͼ��ʾ��

��2�����뷴Ӧ��ǰ����μ������װ�õ������ԣ�______��3��װ��B��ʢ�ŵ��Լ���______��������______��
��4����ʵ����ͨ���Ʒ��У�װ��A������Ϊ����______����ķ���װ�ã�����ţ���
��CH2�TCH2 ��H2S ��CH4 ��CH��CH ��H2
��5��ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4����CO����CO2����O2ʱ�����ڱ������β����������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڣ�

��6����֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸��� Һ�ܶ�Ϊ1.44g/mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ______��
��1���������CO2��NaOH��Һ��Ӧ����NaHCO3���䷴ӦΪNaOH+CO2�TNaHCO3������ʽ����Ӧ�����κ�ˮ���䷴ӦΪNaHCO3+NaOH�TNa2CO3+H2O��
�ʴ�Ϊ��NaOH+CO2�TNaHCO3��NaHCO3+NaOH�TNa2CO3+H2O��
��2����װ�ò�©��ʱ��ͨ���ı������������Ըı�ѹǿ���ʴ𰸣��õ��ɼм�סA��B���Ӵ����ȼ��A�����ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿ�����ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����Ҳ��һ�μ��A��B�������ԣ����Ӻ��ձ�����齺����ֹˮ�м�ס��Ȼ���©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©������
��3����CO2�к���HCl��Ҫ�õ�������CO2�������ȥHCl��ͬʱ�����Լ�����CO2��Ӧ�������ñ���NaHCO3��Һ�������ñ���Na2CO3��Һ���ʴ𰸣�������������Һ������HCl���壻
��4�����Ʊ�CO2��H2S��CH��CH��H2װ�õ��ص㣺����+Һ������壬���Ʊ�CH2�TCH2 װ�õ��ص㣺Һ��+Һ��
���壬�Ʊ�CH4װ�õ��ص㣺����+����
���壬�ʴ𰸣��ڢܢݣ�
��5�����ж�����������β����������CH4��CO2��O2����Ҫ������CO��Ȼ��Ҫ��������������Һ�������գ�����ȼ�շ�����������ߵ�װ����������Ҫ��������˵�����弫������ˮ���磺NH3��HCl �ұߵ�װ�ú�����������û�з�������˵�����岻������ˮ���磺Cl2��H2S���ʴ𰸣��٢ۣ��ڢ�
��6��50mLNaOH��Һ��n��NaOH��=
=0.72mol��
NaOH+CO2�TNaHCO3
0.36mol 0.36mol
NaHCO3 +NaOH�TNa2CO3 +H2O
0.36mol 0.36mol 0.36mol
��Na2CO3�����ʵ���Ũ��Ϊ��C=
=
=7.2 mol/L��
�ʴ𰸣�7.2 mol/L��
�ʴ�Ϊ��NaOH+CO2�TNaHCO3��NaHCO3+NaOH�TNa2CO3+H2O��
��2����װ�ò�©��ʱ��ͨ���ı������������Ըı�ѹǿ���ʴ𰸣��õ��ɼм�סA��B���Ӵ����ȼ��A�����ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿ�����ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����Ҳ��һ�μ��A��B�������ԣ����Ӻ��ձ�����齺����ֹˮ�м�ס��Ȼ���©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©������
��3����CO2�к���HCl��Ҫ�õ�������CO2�������ȥHCl��ͬʱ�����Լ�����CO2��Ӧ�������ñ���NaHCO3��Һ�������ñ���Na2CO3��Һ���ʴ𰸣�������������Һ������HCl���壻
��4�����Ʊ�CO2��H2S��CH��CH��H2װ�õ��ص㣺����+Һ������壬���Ʊ�CH2�TCH2 װ�õ��ص㣺Һ��+Һ��
| ���� |
| ���� |
��5�����ж�����������β����������CH4��CO2��O2����Ҫ������CO��Ȼ��Ҫ��������������Һ�������գ�����ȼ�շ�����������ߵ�װ����������Ҫ��������˵�����弫������ˮ���磺NH3��HCl �ұߵ�װ�ú�����������û�з�������˵�����岻������ˮ���磺Cl2��H2S���ʴ𰸣��٢ۣ��ڢ�
��6��50mLNaOH��Һ��n��NaOH��=
| 50mL��1.44g/mL��40% |
| 40g/mol |
NaOH+CO2�TNaHCO3
0.36mol 0.36mol
NaHCO3 +NaOH�TNa2CO3 +H2O
0.36mol 0.36mol 0.36mol
��Na2CO3�����ʵ���Ũ��Ϊ��C=
| n |
| v |
| 0.36mol |
| 0.05L |
�ʴ𰸣�7.2 mol/L��

��ϰ��ϵ�д�
�����Ŀ
 (1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��
(1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��