��Ŀ����
���ѿ�������ơ�
��1����������֭�������ǵķ����ǣ������мӼ�������ԣ��ټ������Ʊ���Cu��OH��2�����ȣ���������_______________________________________��
��2����������ƹ����У�������ת��Ϊ�ƾ��Ĺ������£�����������л�ѧ����ʽ��
C6H12O6�������ǣ�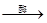 2________+2C2H5OH
2________+2C2H5OH
��3�����Ѿ��ܷⴢ�����������������ζ��������Ҳ����ͨ����ѧʵ�����Ʊ���ʵ��������ͼ��ʾװ���Ʊ�����������
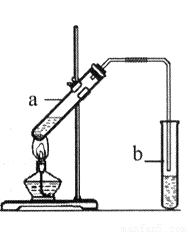
���Թ�a���������������Ļ�ѧ����ʽ��________________________________��
���Թ�b��ʢ�ŵ��Լ��DZ���_________________��Һ��
��ʵ�鿪ʼʱ���Թ�b�еĵ��ܲ�����Һ���µ�ԭ����___________________��
����������Թ�b�����ɵ�������������Ҫ�õ���������____������ţ���
a. ©�� b. ��Һ©�� c. ����©��
��1��������ɫ������2��CO2
��3����CH3COOH+C2H5OH CH3COOC2H5+H2O��2�֣�ûд����ſ�1�֣�
CH3COOC2H5+H2O��2�֣�ûд����ſ�1�֣�
��Na2CO3 �۷�ֹ��Һ���� ��b
��������
�����������1���������к���ȩ�����ܱ����Ƶ�������ͭ����Һ����������ʵ�������Dz�����ɫ������
��2������̼ԭ�Ӻ���ԭ���غ��֪������һ����������CO2��
��3������Ũ����������£��Ҵ������ᷢ��������Ӧ����������������Ӧ�Ļ�ѧ����ʽ��CH3COOH+C2H5OH CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
�������Ҵ������ᶼ���ӷ��ģ��������ɵ����������к����Ҵ������ᣬ��ȥ�Ҵ���������Լ��DZ��͵�̼������Һ��
�������Ҵ������ᶼ�Ǻ�ˮ���ܵģ����ֱ�Ӳ���ˮ�����գ������������������Թ�b�еĵ��ܲ�����Һ���µ�ԭ���Ƿ�ֹ��Һ������
����������������ˮ����Һ���ɣ���Ҫ�õ��������Ƿ�Һ©������ѡb��
���㣺���⿼�������ǵ����ʣ����������Ʊ�ʵ����й�̽��

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�

 2________+2C2H5OH
2________+2C2H5OH