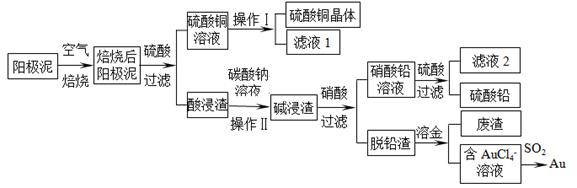
��Ŀ����
��14�֣���ҵ��SnSO4��һ����Ҫ�������Σ��㷺Ӧ���ڶ�����ҵ�����Ʊ�·�����£�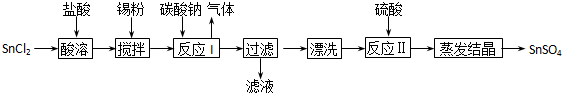
��ʾ������֪�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��
����֪Ksp[Sn(OH)2] ��1.0��10-26
�ش��������⣺
��1��SnCl2�����������ˮֱ���ܽ��ԭ����__________������Sn�۵�������_________��
��2����ӦI���ɵij���ΪSnO��д���÷�Ӧ�����ӷ���ʽ___________________________��
��3����������Ѿ���Ưϴ���ɾ��ķ���__________________________________________��
��4����Ӧ�����������֮һ�ǿ�����Һ��pH������Һ��c(Sn2+)��1.0mol?L-1����Ӧ������ҺpH_____��
��5�����������£�SnSO4����������˫��ˮȥ��������д��������Ӧ�����ӷ���ʽ____________��
��1������Sn2+ˮ�� ��2�֣� ��ֹSn2+��������2�֣���2��Sn2+ + CO32-��SnO�� + CO2����3�֣�
��3��ȡ���һ��ϴ��Һ�������м���AgNO3��Һ������������˵��ϴ�Ӹɾ�����2�֣�
��4��С��1�� ��2�֣� ��5��Sn2+ + H2O2 + 2H+��Sn4+ + 2H2O ��3�֣�
���������������1��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ƽ��Sn Cl2+H2O Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
��2����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�û�б仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼�����ӷ���ʽΪ��Sn2++CO32-�TSnO��+CO2����
��3�����������������������ӣ���˼�������Ѿ���Ưϴ���ɾ��ķ����ǣ�ȡ���һ��ϴ��Һ�������м���AgNO3��Һ������������˵��ϴ�Ӹɾ���
��4������ Ksp[Sn��OH��2]��1.0��10-26��c��OH-��2��c��Sn2+������c��Sn2+����1.0mol?L-1���˿ɵ�c��OH-����10-13mol/L��c��H+����0.1mol/L����pHС��1��Sn2+��ȫ������
��5�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ�Sn2+�ױ�����ΪSn4+����������ԭΪˮ�����ӷ���ʽΪ��Sn2++H2O2+2H+�TSn4++2H2O��
���㣺����Թ������̵����⡢���ʵķ����ᴿ���Ķ���Ŀ��ȡ��Ϣ�����������û�ѧ������д�����ù�ϵʽ���еļ����

������ʵ��װ�úͷ���������Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ��� 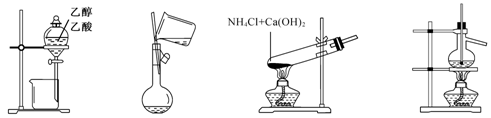
ͼ1 ͼ2 ͼ3 ͼ4
| A����ͼ1��ʾװ�÷����Ҵ������� |
| B����ͼ2��ʾ��װ��������ƿ��ת��Һ�� |
| C����ͼ3��ʾ��װ���Ʊ��������� |
| D����ͼ4��ʾ��װ�÷���ʯ�� |
��֪�ס��ҵ�ijЩ�������£�
| ���� | �ܶ�/(g��mL��1) | �е� | �ܽ��� |
| �� | 0.893 | 78.5�� | ������ˮ�������� |
| �� | 1.220 | 100.7�� | ������ˮ�����ڼ� |
A.���� B.���� C.��Һ D.����
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ� Fe2����Mg2����Al3����Ba2����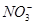 ��
��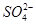 ��Cl����I����
��Cl����I���� ��ȡ����Һ����ʵ�飺
��ȡ����Һ����ʵ�飺
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�ɴ��жϣ�
(1)��Һ�п϶������ڵ������� ����Һ�п϶����ڵ������� ��
(2)�����ʵ����֤���п��ܴ��ڵ������ӵķ���(д��������������) ��
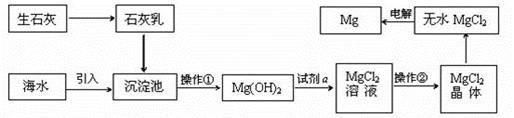
���������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������SiO2��Al2O3�����ʣ��Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7���Ĺ�������ͼ��ʾ��
��֪���� NaFeO2��ˮǿ��ˮ�⡣
�� Cr2O72��+ H2O 2CrO42��+ 2H+
2CrO42��+ 2H+
��1��K2Cr2O7��CrԪ�صĻ��ϼ��� ��
��2����Һ1�ijɷֳ�Na2CrO4��Na2SiO3�⣬�����У��ѧʽ�� ��
��������1�к��ɫ���ʷ�Ӧ�Ļ�ѧ����ʽ�ǡ�
��3������Һ2ת��ΪNa2Cr2O7��ҺӦ��ȡ�Ĵ�ʩ�ǡ�
��4�����ո���������Na2CrO4��NaFeO2��Ӧ�Ļ�ѧ����ʽ�� ��
��5��Ī������һ�ֳ����ζ�������Na2CrO4 Ϊָʾ�����ñ���������Һ�ζ�����Һ�����вⶨ��Һ��Cl����Ũ�ȡ���֪��
| ���� ���� | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| Ksp | 1��34��10��6 | 7��1��10��7 | 1��1��10��8 | 6��5��10��5 | 1��0��10��6 |
�ٵζ��յ�������ǡ�
������AgNO3��Һ�ζ�NaSCN��Һ����ѡΪ�ζ�ָʾ�����ǣ�ѡ���ţ�
A��NaCl B��K2CrO4 C��BaBr2
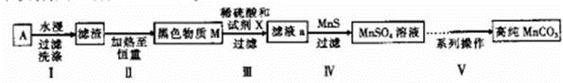

 ���Ҵ���
���Ҵ���