��Ŀ����
��ÿ��2�֣���6�֣��Ȼ�ѧ����ʽ�еĦ�Hʵ����������ѧ�е�һ���������������ʱ䣬����ֵ�ͷ����뷴Ӧ�����������������йأ�Ҳ�뷴Ӧ���������ļ����йء�
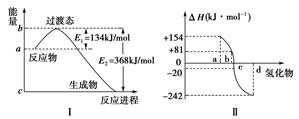
(1)����ͼ����ʾ��ʾ����NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��________________________��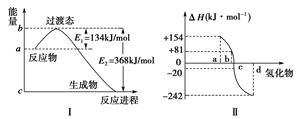
(2)ͼ���ʾ����Ԫ���е��������������������⻯��ʱ���ʱ����ݣ������ʱ����ݿ�ȷ��a��b��c��d�ֱ��������Ԫ�أ���д��������������ѧ��̬�£������ֽⷴӦ���Ȼ�ѧ����ʽ��_________________________________________________��
(3)��֪��
��Fe2O3(s)��3CO(g)===2Fe(s)��3CO2(g)��
��H����25 kJ��mol��1��
��3Fe2O3(s)��CO(g)===2Fe3O4(s)��CO2(g)��
��H����47 kJ��mol��1��
��Fe3O4(s)��CO(g)===3FeO(s)��CO2(g)��
��H��19 kJ��mol��1
��д��CO��ԭFeO���Ȼ�ѧ����ʽ��
________________________________________________________________________��
(1)NO2(g)��CO(g)===NO(g)��CO2(g)����H����234 kJ��mol��1
(2)H2Se(g)===Se(g)��H2(g)����H��+81 kJ/mol
(3)FeO(s)��CO(g)===Fe(s)��CO2(g)����H����11 kJ��mol��1
���������������1����ȷ��д�Ȼ�ѧ����ʽ��?Ҫָ����Ӧʱ���¶Ⱥ�ѹǿ��?����ʽ�����еķ�Ӧ��Ͳ��ﶼҪ������ע�������ڷ�Ӧʱ��״̬��?д����Ӧ���ʱ䣬ע���ʱ�������š���2������Ԫ�������ɿ�֪����Ԫ�ص��������������������⻯��ʱ���ױ��ѣ���Ӧ�ɷ��ȷ�Ӧ��Ϊ���ȷ�Ӧ������a--�ڣ�b--����c--��d--������ͼ�ɿ�����������Ӧ���ʱ�Ϊ+80KJ/mol����3����?��3����?+?��2���ٳ���6���ɵõ�CO��ԭFeO���Ȼ�ѧ����ʽ�ͷ�Ӧ�ʱ�
���㣺�Ȼ�ѧ����ʽ����д����ѧ��Ӧ�ʱ���жϡ���ѧ��Ӧ�ȵļ���
�������������ڱȽϻ���������⣬�Ȼ�ѧ֪ʶ�ڸ߿���ռ�бȽ���Ҫ��λ��