��Ŀ����
(1)�뽫�����������ʣ�KBr��Br2��I2��KI��K2SO4�ֱ��������к����ϣ����һ��δ��ƽ�Ļ�ѧ����ʽ��
KBrO3�� ��H2SO4���� �� �� �� ��H2O��
(2)����û�ѧ����ʽ��I2��KBr�Ļ�ѧ�������ֱ���8��1�����Br2�Ļ�ѧ�������� ��
���뽫��Ӧ��Ļ�ѧʽ����ƽ��Ļ�ѧ����������������Ӧ��λ���У�
KBrO3�� �� H2SO4����������
����ת��10 mol���ӣ���Ӧ������I2�����ʵ���Ϊ ��
(1)KI I2 Br2 K2SO4 KBr
(2)��1 ��3 16KI 9 ��5 mol
����

������NO�ķ�Ӧԭ��Ϊ��2CO��2NO=N2��2CO2�йظ÷�Ӧ��˵������ȷ���� ( )
| A����Ӧ��COΪ������ |
| B����Ӧ��NO����ԭ |
| C���ڷ�Ӧ����1 mol N2ʱ��ת�Ƶĵ���Ϊ4 mol |
| D��CO��NO������ɫ�ж����� |
���û��ϼۺ���������Ʋ����ʵ������ǻ�ѧ�о�����Ҫ�ֶΡ�
��1���ӻ��ϼ۵ĽǶȿ���Ԥ�����ʵ����ʡ�
�ٽ� ͨ������
ͨ������ ��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��
��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��
| A��S2- | B��S | C��SO32- | D��SO42- |
��ѡȡ���ʵ��Լ�֤��Na2SO3���л�ԭ�ԣ� ��д���÷�Ӧ�����ӷ���ʽΪ�� ��
��2�������ʷ���ĽǶȿ����Ʋ����ʵ����ʡ�
����֪����ʯ��MgO��Al2O3��SiO2��Fe2O3��ɡ��������ڼ������������ ��
����ȡһ������ʯ��������ʵ�飺
I���Ƚ������ڹ����������С����ˣ���������Ҫ�ɷ��� ��
II��������Һ�м���NaOH��Һ�����������ˣ������е���Ҫ�ɷ��� ��
Ϊ֤��Fe3+���н�ǿ�������ԣ���ͬѧ��������ʵ�飺��CuƬ����0.5mol/L Fe(NO3)3��Һ�У��۲쵽CuƬ���ܽ⣬��Һ�ɻ�ɫ��Ϊ����ɫ���ɴ˼�ͬѧ�õ�Fe3+���н�ǿ�����ԵĽ��ۡ�
��ͬѧ����˲�ͬ�Ŀ�������Fe(NO3)3��Һ�������ԣ��ڴ�����������NO3-Ҳ������Cu���������ʵ�����̽������֪��
| ˮ�ⷴӦ | ƽ�ⳣ����K�� |
Fe3+ + 3H2O  Fe(OH)3 + 3H+ Fe(OH)3 + 3H+ | 7.9 �� 10-4 |
Fe2+ + 2H2O  Fe(OH)2 + 2H+ Fe(OH)2 + 2H+ | 3.2 �� 10-10 |
Cu2+ + 2H2O  Cu(OH)2 + 2H+ Cu(OH)2 + 2H+ | 3.2 �� 10-7 |
��ش𣺣�1��ϡ�����Cu��Ӧ�Ļ�ѧ����ʽΪ ��
��2�����������ṩ���Լ���������ͬѧ���ʵ�鷽����ơ�
�Լ���0.5mol/L Fe(NO3)3��Һ��CuƬ������pH��ֽ��0.5��5.0������������Һ��ϡ���ᡣ
������ ��
��3����ͬѧ�ֱ�ʵʩ�˼ס�����λͬѧ��ʵ�鷽��������ʵ���������pH�Ƽ����ҺpH�ı仯��ʵ���¼���¡�
| ʵ������ | ʵ������ |
| ��ͬѧ��ʵ�鷽�� | ��Һ�������ɫ�� pH�������� |
| ��ͬѧ��ʵ�鷽�� | ����������pHû�����Ա仯�� |
�پ�ʵ������д��������Ӧ�����ӷ���ʽ�� ��
�ڵ���ʵ���������ҺpH���������Ŀ���ԭ���� ��
�۽�����ͬѧ��ʵ������ ��
��4��������Ƹ������е�ʵ�鷽����������ͬѧ�ﵽʵ��Ŀ�ģ� ��
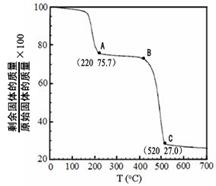
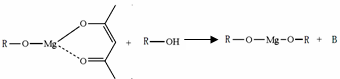
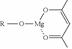 �����ɷ������·�Ӧ��
�����ɷ������·�Ӧ��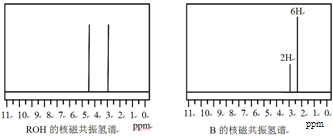

 �� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
�� ��Ӧ�ٵĻ�ѧ����ʽΪ ��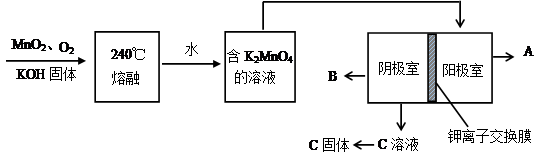
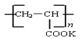 �����۱�ϩ��ص���Ľṹ��ʽΪ ��
�����۱�ϩ��ص���Ľṹ��ʽΪ ��