��Ŀ����
��2009?���ݶ�ģ��ˮ������֮Դ��2005��12�·�������ʡ�ı���ˮ��Ⱦ�¼��ٴ��������DZ���ˮ��Դ�ı�Ҫ�Ժͽ����ԣ�
��1���ݱ���������ˮ��Ⱦ�¼�������ijұ�����豸�����ڼ��ŷŵķ�ˮ���£��Ʋ�����Ҫ��Ⱦ����
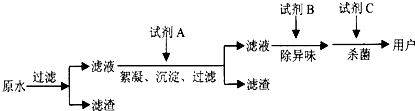
��2������ˮ������������ͼ�����е��Լ�A��B��C�ֱ����ѡ��
��3����Ƴ��ŷŵķ�ˮ�к�������Cr2O72-��ʽ���ڣ������õ��ķ������������ʱ������Ϊ���������ɵ�Fe2+��Cr2O72-��ԭΪCr3+������������ʱ���ɵ�OH-�γ����������������ȥ��
�������缫�Ϸ����ķ�Ӧ�ֱ�Ϊ������
����Һ�з������ܷ�ӦΪ�������ӷ���ʽ��ʾ��
��4����ѧ��������COD����ˮ�������Ŀ�����Ŀ��һ����������ˮ�л�ԭ����Ⱦ�����Ҫָ�꣮COD��ָ��ǿ���������ҹ�����K2Cr2O7������һ����ˮ��ʱ���ĵ����������������������O2��Ϊ������ʱ��1Lˮ��������O2��������mg?L-1������ȡij����ˮ��20.00mL����Ӧ����10.00mL0.0400mol?L-1K2Cr2O7��Һ����Ӧ��ת��ΪCr3+������ˮ����CODΪ
��1���ݱ���������ˮ��Ⱦ�¼�������ijұ�����豸�����ڼ��ŷŵķ�ˮ���£��Ʋ�����Ҫ��Ⱦ����
�ؽ�������
�ؽ�������
������к���ˮ�Ѿ��ں�������ɢ��Ϊ�˾�������Σ�����Ժ�ˮ�Ĵ���Ӧ�ò�ȡ�Ĵ�ʩ����������ˮԴϡ��
��������ˮԴϡ��
��2������ˮ������������ͼ�����е��Լ�A��B��C�ֱ����ѡ��
���������������ȣ�
���������������ȣ�
������̿
����̿
��Cl2����ClO2��O3�ȣ�
Cl2����ClO2��O3�ȣ�
��
��3����Ƴ��ŷŵķ�ˮ�к�������Cr2O72-��ʽ���ڣ������õ��ķ������������ʱ������Ϊ���������ɵ�Fe2+��Cr2O72-��ԭΪCr3+������������ʱ���ɵ�OH-�γ����������������ȥ��
�������缫�Ϸ����ķ�Ӧ�ֱ�Ϊ������
Fe-2e-=Fe2+
Fe-2e-=Fe2+
������2H++2e-=H2��
2H++2e-=H2��
������Һ�з������ܷ�ӦΪ�������ӷ���ʽ��ʾ��
6Fe2++Cr2O72-+10 OH-+7H2O=6Fe��OH��3��+2Cr��OH��3��
6Fe2++Cr2O72-+10 OH-+7H2O=6Fe��OH��3��+2Cr��OH��3��
����4����ѧ��������COD����ˮ�������Ŀ�����Ŀ��һ����������ˮ�л�ԭ����Ⱦ�����Ҫָ�꣮COD��ָ��ǿ���������ҹ�����K2Cr2O7������һ����ˮ��ʱ���ĵ����������������������O2��Ϊ������ʱ��1Lˮ��������O2��������mg?L-1������ȡij����ˮ��20.00mL����Ӧ����10.00mL0.0400mol?L-1K2Cr2O7��Һ����Ӧ��ת��ΪCr3+������ˮ����CODΪ
960
960
mg?L-1����������1��������ұ��������ˮ��ɵ���Ⱦ��һ�����ؽ���������ɵģ����Բ���ϡ�ͷ���������
��2�����ݼ����Լ���Ŀ��ѡ��������Լ�����ɱ��ʹ��ǿ�����Ե��Լ���
��3������������������Ӧ������������ԭ��Ӧ��
����Һ���������ӱ��ظ���������������ӣ���Һ�������������ӣ�������������������������������
��4�����ݵ����غ���㣮
��2�����ݼ����Լ���Ŀ��ѡ��������Լ�����ɱ��ʹ��ǿ�����Ե��Լ���
��3������������������Ӧ������������ԭ��Ӧ��
����Һ���������ӱ��ظ���������������ӣ���Һ�������������ӣ�������������������������������
��4�����ݵ����غ���㣮
����⣺��1��������ұ��������ˮ��Ⱦ��Ӧ�����ؽ�����������ģ�������������û����Ⱦ��ˮ����ϡ�ͣ������ؽ�������Ũ�ȣ�
�ʴ�Ϊ���ؽ������ӣ���������ˮԴϡ�ͣ�
��2�������Լ�A�ӿ�ˮ�в����Թ���С�����ij���������ѡ������������������
�Լ�B��ȥˮ����ζ������ѡ�����̿��
�Լ�C��ɱ�������ã�ͨ��ѡ����������ClO2��O3�ȣ�
�ʴ�Ϊ�����������������ȣ��� ����̿�� Cl2����ClO2��O3�ȣ���
��3����������ʧȥ���ӣ��缫��Ӧ�ǣ�Fe-2e-=Fe2+������ˮ����������ӵõ����ӣ��缫��ӦΪ��2H++2e-=H2����
�ʴ�Ϊ��Fe-2e-=Fe2+��2H++2e-=H2����
����Һ�з����ķ�Ӧ�ǣ��������ӱ��ظ�������������������ӣ��ظ���ر���ԭ�ɸ����ӣ������Ӻ����ӽ����Һ�е������������������������������������������Ӧ�������ӷ�Ӧ����ʽ�ǣ�6Fe2++Cr2O72-+10 OH-+7H2O=6Fe��OH��3��+2Cr��OH��3����
�ʴ�Ϊ��6Fe2++Cr2O72-+10 OH-+7H2O=6Fe��OH��3��+2Cr��OH��3����
��4��10.00mL0.0400mol?L-1K2Cr2O7��Һ��ȫ��Ӧת�Ƶ������ʵ����ǣ�0.01L��0.0400mol/L��2��6-3��=2.4��10-3mol����Ҫ�������������ʵ����ǣ�
��2.4��10-3mol=6��10-4mol��1Lˮ�����ĵ������������ǣ�
��6��10-4mol��32g/mol=0.96g=960mg��
�ʴ�Ϊ��960��
�ʴ�Ϊ���ؽ������ӣ���������ˮԴϡ�ͣ�
��2�������Լ�A�ӿ�ˮ�в����Թ���С�����ij���������ѡ������������������
�Լ�B��ȥˮ����ζ������ѡ�����̿��
�Լ�C��ɱ�������ã�ͨ��ѡ����������ClO2��O3�ȣ�
�ʴ�Ϊ�����������������ȣ��� ����̿�� Cl2����ClO2��O3�ȣ���
��3����������ʧȥ���ӣ��缫��Ӧ�ǣ�Fe-2e-=Fe2+������ˮ����������ӵõ����ӣ��缫��ӦΪ��2H++2e-=H2����
�ʴ�Ϊ��Fe-2e-=Fe2+��2H++2e-=H2����
����Һ�з����ķ�Ӧ�ǣ��������ӱ��ظ�������������������ӣ��ظ���ر���ԭ�ɸ����ӣ������Ӻ����ӽ����Һ�е������������������������������������������Ӧ�������ӷ�Ӧ����ʽ�ǣ�6Fe2++Cr2O72-+10 OH-+7H2O=6Fe��OH��3��+2Cr��OH��3����
�ʴ�Ϊ��6Fe2++Cr2O72-+10 OH-+7H2O=6Fe��OH��3��+2Cr��OH��3����
��4��10.00mL0.0400mol?L-1K2Cr2O7��Һ��ȫ��Ӧת�Ƶ������ʵ����ǣ�0.01L��0.0400mol/L��2��6-3��=2.4��10-3mol����Ҫ�������������ʵ����ǣ�
| 1 |
| 4 |
| 1000mL |
| 20mL |
�ʴ�Ϊ��960��
���������⿼����ˮ�������ⶨ���漰����ˮ��������ⷴӦ����ʽ��д��֪ʶ�������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ