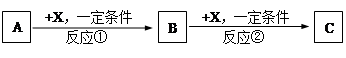��Ŀ����
A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ����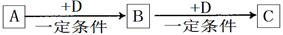
�Իش�
��1����D�Ǿ��������Եĵ��ʣ������ڶ����ڵ��������Ԫ��AΪ ����Ԫ�ط��ţ���
��2����D�ǽ������ʣ�D�ڳ�ʪ�Ŀ���������������ʴ��C��Һ�ڱ���ʱӦ�����������D��ֹ����ʣ�������D��C��Һ�ڿ����б��ʵ����ӷ���ʽΪ ����D���Ȼ����ˮ��Һ���ɲ����ղ����� ��
��3����A��B��C��Ϊ��������Ҿ����ؿ��к�����ߵĽ���Ԫ��E������Һ��A��C��Ӧ����B����д��Bת��ΪC�����п��ܵ����ӷ���ʽ ��
��4�����ڣ�1�����Ƴ���A������ڣ�3����E���ʵĻ����11.9gͶ��һ������ˮ�г�ַ�Ӧ��A��E��û��ʣ�࣬���ռ�����״���µ�����vL����������Һ����μ���Ũ��Ϊ2mol?L-1��H2SO4��Һ����100mLʱ��ɫ�����ﵽ���������v= ��
��1��Na
��2��4Fe2����O2+4H+��4Fe3��+2H2O�� Fe2 O3
��3��Al(OH)3��3H����Al3����3H2O��Al(OH)3��OH����AlO2����2H2O
��4��7.84L
���������������1�������������Եĵ���ΪO2��Cl2��AΪ�����ڽ���Ԫ�أ�����ת����ϵ����A��Na��B��Na2O��C��Na2O2��D��O2��
��2��D���ڳ�ʪ�Ŀ���������������ʴ�Ľ������ʣ�����Ԫ�أ���C�к���Fe2+����FeCl3��ˮ��Һ���ɲ����գ�����Fe3+��ˮ���HCl�Ļӷ������յõ��IJ�����Fe2O3��
��3���ؿ��к�����ߵĽ���Ԫ����������A��B��C�ת����ϵ��֪B��Al(OH)3��A��C�зֱ���Al3+��AlO2��������ȷ�Ͼ��庬���ĸ����ӣ���Bת��ΪC��������Al(OH)3ת��ΪAl3������Al(OH)3ת��ΪAlO2����
��4������100 mL 2mol?L-1��H2SO4��Һʱ���������������ʱ��Һ�н���Na2SO4����ԭ���غ��֪��n(Na)=2n(Na2SO4)=2n(H2SO4)="2" ��0.1 L��2mol?L-1="0.4" mol����ԭ����������Ƶ�����Ϊ0.4 mol��23 mol·L��1=9.2g����������Ϊ11.9g��9.2g=2.7g���ʷ�Ӧ����H2�����Ϊ(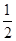 ��0.4mol+
��0.4mol+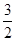 ��
��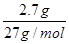 )��22.4L/mol="7.84" L��
)��22.4L/mol="7.84" L��
���㣺�ƵĻ�ѧ���ʣ���ѧ����ʽ���йؼ��㣻���Ļ�ѧ����

 ������������ϵ�д�
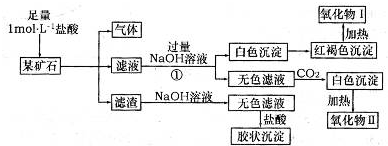
������������ϵ�д������ڵ�����Ԫ��A��B��C��D��E,ԭ��������������A��B��C����Ԫ�ص��Ӳ���֮����5��A��B��Ԫ��ԭ������������֮�͵���CԪ��ԭ������������;BԪ��ԭ��������Ӳ��ϵĵ����������ĵ��Ӳ�����2��,A��D�����γ�ԭ�Ӹ����ȷֱ�Ϊ1��1��2��1������Һ̬������;E�������ھ���ˮ�ʡ�
��ش�:
(1)д��D��Ԫ�����ڱ��е�λ���� ,
E��ԭ�ӽṹʾ��ͼ��������������������
���п�����֤C��D��Ԫ��ԭ�ӵõ�������ǿ����ʵ����ʵ����������(��д���)��
A.�Ƚ�������Ԫ�ص���̬�⻯��ķе�
B.�Ƚ�ֻ��������Ԫ�����γɵĻ������еĻ��ϼ�
C.�Ƚ�������Ԫ�ص���̬�⻯����ȶ���
D.�Ƚ�������Ԫ�صĵ������������ϵ�����
(2)��A��B����Ԫ����ɵ���Ļ�����,д�������ʽ����������
(3)����A��B��C��D����Ԫ����ɵļס������ֻ�����,���ȿ��������ᷴӦ�ֿ�����NaOH��Һ��Ӧ,��Ϊ����,�仯ѧʽΪ��������,��Ϊ��Ȼ�߷��ӻ������ˮ�����,����ͬ����������Է���������С��,��ṹ��ʽΪ������������������������
(4)��̬��������ҽѧ������Ҫ����;,������Fe3O4�Ǵ������е���Ҫ����,���Ʊ����̿ɼ�ʾ����:
�ٽ�������CA3ͨ������ʵ�����FeSO4��Fe2(SO4)3�Ļ����Һ��,�������ּ�,д���÷�Ӧ���̵��ܵ����ӷ���ʽ�� ��
��������Ӧ���ɵ����ּ��������,�õ�Fe3O4��
(5)��֪�±�����:
| ���� | Fe(OH)2 | Fe(OH)3 |
| Ksp/25 �� | 2.0��10-16 | 4.0��10-36 |
��ʹ���Һ��FeSO4��Fe2(SO4)3��Ũ�Ⱦ�Ϊ2.0 mol��L-1,����Һ��c(OH-)���ô�����������mol��L-1��
X��Y��Z��W��Ԫ�����ڱ�ǰ�����ڵij���Ԫ�أ��������Ϣ���±�:
| X | X��һ�ֺ��ص�������Ϊ56��������Ϊ30 |
| Y | ��ˮ��Ԫ�غ�����ߵĽ���Ԫ�� |
| Z | ���³�ѹ�£�Z�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
| W | �۵��ӵ��Ų�ʽΪ3s23p3 |
��1��ZԪ����Ԫ�����ڱ��е�λ��Ϊ ��Z�ĵ縺�Ա�W�� �����С������
��2��XԪ���Ļ�̬ԭ�ӵ����Ų�ʽ�� _ ������___���˶�״̬��ͬ�ĵ��ӡ���Ԫ�صİ�ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ ��
��3��Y�ĵ����ڹ�ҵ����;�㷺����ҵ����YW��ȡY���ʵĻ�ѧ����ʽΪ ��
��4�����ʵ����֤Z�ͣķǽ����Ե�ǿ���� ��
A��B��C��D��ԭ��������������Ķ���������Ԫ�أ�A��C��Ԫ�����ڱ��е����λ����ͼ��AԪ��������������ϵĵ�����֮��Ϊ3��BΪ�ؿ��к������Ľ���Ԫ�ء�
| A | |
| | C |
��1��Dԭ�ӽṹʾ��ͼΪ_____________��
��2����C�ĵͼ�̬�������ͨ�뵽D���ʵ�ˮ��Һ��ʹ֮��ɫ�������˼�________�ԣ�д���÷�Ӧ�����ӷ���ʽ_____________________��
��3��A������������Ӧ��ˮ�������ң��ֽ�����Cu���뵽100 mL 8.0 mol/L�ҵ�Ũ��Һ�У���ַ�Ӧ�����ռ���6.72L����״�������壬�������ijɷ���_______________����ԭ��ʧ������Ϊ_________________��
��4��������������B���ʷֱ���뵽�������Ũ�ȵ������NaOH��Һ�У���ַ�Ӧ��������������Ϊ____________________��������Ӧ�����õ���Һ��ϣ������ɰ�ɫ������������Ӧ�����ӷ���ʽΪ___________________________________________________��B���ʱ��������Ĥ����NaOH��Һ��ȥ��д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________________________________��
X��Y��Z��Q��MΪ�����Ķ�����Ԫ�أ���ԭ���������������й���Ϣ���±�:
| X | ��ֲ����������ȱ�ٵ�Ԫ�أ��ǵ����ʵ���Ҫ�ɷ� |
| Y | �ؿ��к����ӵ�һλ |
| Z | ����������ԭ�Ӱ뾶��� |
| Q | �����д���ʹ����Ͻ���Ʒ����ҵ�Ͽ��õ����������ķ����Ʊ� |
| M | ��ˮ�д���������Ԫ��֮һ������������ϼ��븺�۵Ĵ�����Ϊ6 |
��1��X����̬�⻯��Ĵ���������������˵���������ʳ��������µļ������������⣬��д������̬�⻯��ĵ���ʽ____________��
��2����֪37Rb��53I��λ�ڵ������ڣ��ֱ���Z��Mͬһ���塣�����й�˵����ȷ����____________������ţ���
A��ԭ�Ӱ뾶�� Rb>I
B��RbM�к��й��ۼ�
C����̬�⻯�����ȶ��ԣ�M>I
D��Rb��Q��M������������Ӧ��ˮ�����������������Ӧ
��3��������QX�����Ժã�������ϵ��С�������õ����ȳ�����ϡ������ڽ�����ʴ������ǿ���������������������Ͻ�������������ϡ��йػ�����QX���Ʊ�����ѧ�������£������������ݾ����ۺ�Ϊ25�桢101.3 kPa�����µ���ֵ��
����Q��X�ĵ�����800 ~ 1000���Ƶã�ÿ����1 mol QX������a kJ��������
����Q���������̿��X�ĵ�����1600 ~ 1750������QX��ÿ����1 mol QX������18 g̼������b kJ��������
�����������Ϣд����������Q�����������̿��Ӧ����Q���ʺ�CO���Ȼ�ѧ����ʽ________________��
��4��X��Y��ɵ�һ����ɫ������������Ϊ����ɫ������״����40 �̸���ɫ������15 ������ͨ��һ��Ũ�ȵ�NaOH��Һ�У�ǡ�ñ���ȫ���գ�ͬʱ���������Ρ���д���÷�Ӧ�����ӷ���ʽ ��
���������У�SԪ�صĻ��ϼ�Ϊ+6����
| A��S | B��H2SO4 | C��SO2 | D��H2S |