��Ŀ����
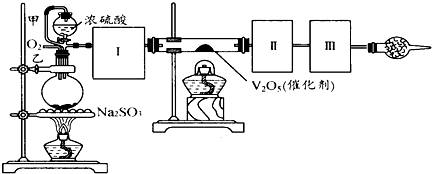
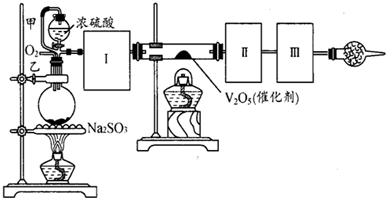
����ͼװ�ÿ��Խ��вⶨSO2ת����SO3��ת���ʵ�ʵ�顣��֪SO3���۵���16.8�棬�е���44.8�档��֪����װ�������漰��Ӧ�Ļ�ѧ����ʽΪ��
Na2SO3(s) + H2SO4(85%)==Na2SO4 + H2O + SO2��
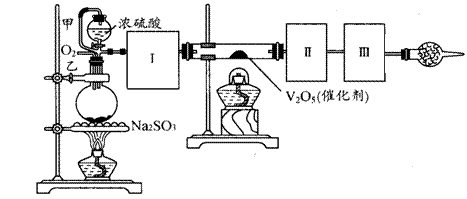 |
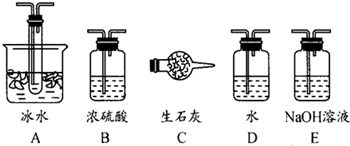
��1������ʵ����Ҫ��Ӧ���ڢ����Ӻ��ʵ�װ�á������ͼA��Eװ����ѡ�����ʺ�װ�ò����������������Ŀո��С�
�����ӵ�װ�÷ֱ��� _______ �� _____ �� _______ ��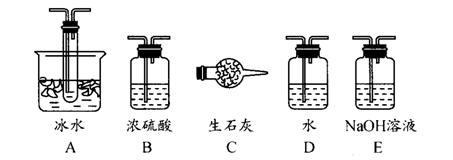
��2�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ�����ڼ��ȴ�����μ�Ũ�����˳���У�Ӧ��ȡ�IJ����� �����ô�������ƿʱ��SO2��ת���ʻ� (����ߡ��������䡱���͡�)
��3����һС����ʵ���з��֣�SO2����������������º���ʵ���������ԣ����ֲ��������������⣬�����Ʋ���ܵ�ԭ��˵����Ӧ����֤���������Բ���������
��ԭ�� ����֤����
��ԭ�� ����֤����
��ԭ�� ����֤����
��4����SO2ͨ�뺬1.5mol�������Һ�У�������һ��ǿ���һ�����������1.5��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ
��5����amolNa2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ����װ�â�������bg����ʵ����SO2��ת����Ϊ �����ú�a��b�Ĵ���ʽ��д��
(1) B A E��2�֣���д1����1�֣���0�֣����������𰸾����֣�
(2)�ȼ��ȴ����ٵ���Ũ���� ��2�֣� ���� ��1�֣� (3)��ԭ�� Na2SO3���� ��1�֣�����֤���� ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤����Na2SO3���������2�֣���ԭ�� ����Ũ���� ��1�֣�����֤���� �ýྻ������պȡ����������Ϳ��ֽ����ڣ���֤������Һ����Ũ���� ��2�֣��������𰸺���Ҳ���֣�
(4)SO2 + 2HClO3 = H2SO4 + 2ClO2��2�֣� (5)![]() ��������
��������![]() �� 2��
�� 2��
����:

 Na2SO4+SO2��+H2O��ע��80%H2SO4����Ũ��������ԣ�
Na2SO4+SO2��+H2O��ע��80%H2SO4����Ũ��������ԣ�