��Ŀ����
��1���봿ˮ�ĵ������ƣ�Һ����Ҳ���������ĵ��룺2NH3 NH4++NH2- �ݴ��ж����������д������ �� ��
NH4++NH2- �ݴ��ж����������д������ �� ��
��2��������з�Ӧ����ʽ
����Һ����Ͷ��һС������ƣ��ų������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���
��NaNH2����ˮ�ķ�Ӧ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���
�������ڡ�H++OH��=H2O���ķ�Ӧ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���
 NH4++NH2- �ݴ��ж����������д������ �� ��
NH4++NH2- �ݴ��ж����������д������ �� ��| A��Һ���к���NH3��NH4+��NH2-���� |
| B��һ���¶���Һ����C(NH4+)��C(NH2-)��һ������ |
| C��Һ���ĵ���ﵽƽ��ʱC(NH3) = C(NH4+) = C(NH2-) |
| D��ֻҪ�������������ʣ�Һ����C(NH4+) = C(NH2-) |
����Һ����Ͷ��һС������ƣ��ų������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���
��NaNH2����ˮ�ķ�Ӧ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���
�������ڡ�H++OH��=H2O���ķ�Ӧ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���
��1��C��2����2Na+2NH3=H2��+2NaNH2��NaNH2+H2O=NaOH+NH3����NH2��+H2O="OH��+NH3����NH2��+NH4+" =2NH3����NH4Cl+NaNH2=2NH3��+NaCl
����Ҫ������ˮ��ż��ʵ��(ˮ���ӵ��������H+��H2O����γ�H3O+)�Լ�ˮ�ĵ���ƽ��,����Ǩ��Ӧ���ڶ���NH3�������ʶ��NH3���ӵ������H+��NH2��,H+��NH3�������NH4+��Һ���������������NH2����NH4+��һ���¶�������Ũ�ȳ˻�Ϊһ������NH4+������H+��NH2��������OH�����߱�����֪ʶ�Ϳ�˳����ɽ��⡣

��ϰ��ϵ�д�
�����Ŀ
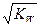
 2XY3(g) ��H����92.6 kJ��mol��1
2XY3(g) ��H����92.6 kJ��mol��1 O2(g)
O2(g) CO(g)�ķ�Ӧ��Ϊl10.5kJ��mol��˵��̼��ȼ����Ϊ110.5kJ��mol
CO(g)�ķ�Ӧ��Ϊl10.5kJ��mol��˵��̼��ȼ����Ϊ110.5kJ��mol