��Ŀ����
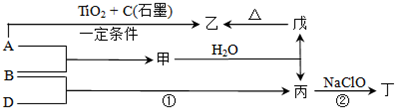
 ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��C��Ϊ�����ڽ������ʣ�C��ˮ��Ӧ����D����������壬D��H����ɫ��Ӧ���ʻ�ɫ����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ������Ӧ���������ɵ�ˮ��������������ȥ��
ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��C��Ϊ�����ڽ������ʣ�C��ˮ��Ӧ����D����������壬D��H����ɫ��Ӧ���ʻ�ɫ����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ������Ӧ���������ɵ�ˮ��������������ȥ����ش��������⣺
��1��B��
AlCl3
AlCl3
��H��NaHCO3
NaHCO3
���ѧʽ������2��д��Eת��ΪG�����ӷ���ʽ
Al��OH��3 +OH-=AlO2-+2H2O
Al��OH��3 +OH-=AlO2-+2H2O
����3��д��H�ڼ����·�Ӧ����F�Ļ�ѧ����ʽ
2NaHCO3
Na2CO3+CO2��+H2O
| ||
2NaHCO3
Na2CO3+CO2��+H2O
��
| ||
������C��ˮ��Ӧ����D����������壬������ӦΪH2��D����ɫ��Ӧ�ʻ�ɫ��ӦΪNaOH����CΪNa����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ��EӦΪ�����������ӦΪAl��OH��3 ����AΪAl��BΪAlCl3 ��FΪNa2CO3��GΪNaAlO2��HΪNaHCO3��������ʵ����ʺ���ĿҪ��ɽ����⣮
����⣺C��ˮ��Ӧ����D����������壬������ӦΪH2��D����ɫ��Ӧ�ʻ�ɫ��ӦΪNaOH����CΪNa����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ��EӦΪ�����������ӦΪAl��OH��3 ����AΪAl��BΪAlCl3 ��FΪNa2CO3��GΪNaAlO2��HΪNaHCO3����
��1�������Ϸ�����֪BΪAlCl3 ��HΪNaHCO3���ʴ�Ϊ��AlCl3 ��NaHCO3��
��2��Al��OH��3Ϊ�������������NaOH��Ӧ����NaAlO2����Ӧ�ķ���ʽΪAl��OH��3 +OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3 +OH-=AlO2-+2H2O��
��3��HΪNaHCO3�������ֽ⣬��Ӧ�ķ���ʽΪ2NaHCO3
Na2CO3+CO2��+H2O��
�ʴ�Ϊ��2NaHCO3
Na2CO3+CO2��+H2O��
��1�������Ϸ�����֪BΪAlCl3 ��HΪNaHCO3���ʴ�Ϊ��AlCl3 ��NaHCO3��
��2��Al��OH��3Ϊ�������������NaOH��Ӧ����NaAlO2����Ӧ�ķ���ʽΪAl��OH��3 +OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3 +OH-=AlO2-+2H2O��
��3��HΪNaHCO3�������ֽ⣬��Ӧ�ķ���ʽΪ2NaHCO3
| ||
�ʴ�Ϊ��2NaHCO3
| ||
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ͻ�ƿ�Ϊ������ܶȡ���ɫ��Ӧ�Լ����ʵ����Եȣ�����ʱע����ᣮ

��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
�����Ŀ
| |||||||||||||||||||
 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����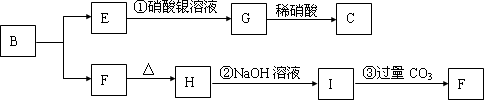
 ��08�Ƹ���ѧ��ģ��(15��)��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ(���ֲ�����ȥ)��
��08�Ƹ���ѧ��ģ��(15��)��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ(���ֲ�����ȥ)��