��Ŀ����
ʵ������һƿ���ܺ���NaCl��Na2SO4��KNO3��K2CO3��K2SO4�е�һ�ֻ������ʵ�Na2SO3��ͨ������ʵ��ȷ������Ʒ�ijɷּ�Na2SO3�������������ƴ���Ʒ6.30 g������6.0 mol��L-1��������������������ɫ����560 mL(��״��)�����ݳ���������Һ�м����Թ�����BaCl2��Һ���õ���ɫ����9.32 g������ɫ�ܲ����۲죬��Һ����ɫ��Ӧ����ɫ�������ʵ����д���пհף�
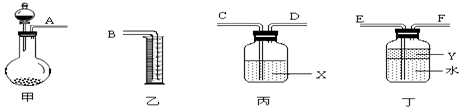
(1)������ͼ��ʾ������װ��������ʵ�飬��������Ҹ���������ȷ����˳��Ϊ_______��_______��_______��________��______��________��X��Һ��_____________�����͵�������___________________________________________________��

(2)����ʱ������������ȷ�ز���ų�����������__________________________________��
(3)��ʵ���м���������Һ�����Ϊ5.00 mL������Ʒ��Na2SO3�����������Ƕ���(д���������)��
(4)������ӷ���ʽ��___________________________________________________��
(5)һ�����е�������ʲô��(д���������)
(1)A E F D C B ŨH2SO4 ��ֹSO2��ˮ�Ӵ�
(2)����Ӧװ����ȴ�����º��������ƶ���Ͳ��ʹ��Ͳ���ƿ��Һ�汣��ˮƽ��Ȼ�����
(3)m(Na2SO3)=![]() ��126 g��mol-1=3.15 g
��126 g��mol-1=3.15 g
w(Na2SO3)=![]() ��100%=50%
��100%=50%
(4)![]() +2H+====SO2��+H2O��Ba2++
+2H+====SO2��+H2O��Ba2++![]() ====BaSO4��
====BaSO4��
(5)��Ϊn(BaSO4)=![]() =0.04 mol
=0.04 mol
����H2SO4Ӧ��BaSO4�����ʵ���Ϊ0.005 L��6.0 mol��L-1=0.03 mol
ԭ�������m(Na2SO4)=(0.04-0.03) mol��142 g��mol-1=1.42 g
m(Na2SO4)+m(Na2SO3)=3.15 g+1.42 g=4.57 g��6.30 g
����Ʒ��Ӧ��NaCl��Na2SO4�������ʡ�
������ͨ����ɫ��Ӧ����ȷ����Ʒ����KNO3��K2CO3��K2SO4���Ƿ�NaCl��Na2SO4��ͨ��ʵ�����ݵĴ����ó����ۡ�

 ��У����ϵ�д�
��У����ϵ�д�