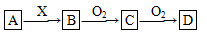ΧβΡΩΡΎ»ί
ΈΣΝΥΧΫΨΩAgNO3ΒΡ―θΜ·–‘ΚΆ»»Έ»Ε®–‘Θ§Ρ≥Μ·―ß–Υ»Λ–ΓΉι…ηΦΤΝΥ»γœ¬ Β―ιΓΘ
Δώ.AgNO3ΒΡ―θΜ·–‘
ΫΪΙβΝΝΒΡΧζΥΩ…λ»κAgNO3»ή“Κ÷–Θ§“ΜΕΈ ±ΦδΚσΫΪΧζΥΩ»Γ≥ωΓΘΈΣΦλ―ι»ή“Κ÷–FeΒΡ―θΜ·≤ζΈοΘ§ΫΪ»ή“Κ÷–ΒΡAgΘΪ≥ΐΨΓΚσΘ§Ϋχ––ΝΥ»γœ¬ Β―ιΘ§Ω…―Γ”Ο ‘ΦΝΘΚKSCN»ή“ΚΓΔK3[Fe(CN)6]»ή“ΚΓΔ¬»Υ°ΓΘ
(1)«κΆξ≥…œ¬±μΘΚ
≤ΌΉς | œ÷œσ | Ϋα¬έ |
»Γ…ΌΝΩ≥ΐΨΓAgΘΪΚσΒΡ»ή“Κ”Ύ ‘Ιή÷–Θ§Φ”»κKSCN»ή“ΚΘ§’ώΒ¥ |
| ¥φ‘ΎFe3ΘΪ |
»Γ…ΌΝΩ≥ΐΨΓAgΘΪΚσΒΡ»ή“Κ”Ύ ‘Ιή÷–Θ§Φ”»κ________Θ§’ώΒ¥ |
| ¥φ‘ΎFe2ΘΪ |
ΓΨ Β―ιΫα¬έΓΩ FeΒΡ―θΜ·≤ζΈοΈΣFe2ΘΪΚΆFe3ΘΪΓΘ
Δρ.AgNO3ΒΡ»»Έ»Ε®–‘
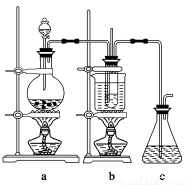
”Οœ¬ΆΦΥυ ΨΒΡ Β―ιΉΑ÷ΟAΦ”»»AgNO3ΙΧΧεΘ§≤ζ…ζΚλΉΊ…ΪΤχΧεΘ§‘ΎΉΑ÷ΟD÷– ’Φ·ΒΫΈό…ΪΤχΧεΓΘΒ±Ζ¥”ΠΫα χΚσΘ§ ‘Ιή÷–≤–ΝτΙΧΧεΈΣΚΎ…ΪΓΘ
(2)ΉΑ÷ΟBΒΡΉς”Ο «________ΓΘ
(3)Ψ≠–ΓΉιΧ÷¬έ≤Δ―ι÷ΛΗΟΈό…ΪΤχΧεΈΣO2Θ§Τδ―ι÷ΛΖΫΖ® «________ΓΘ
(4)ΓΨ≤ι‘ΡΉ ΝœΓΩ Ag2OΚΆΖέΡ©Ή¥ΒΡAgΨυΈΣΚΎ…ΪΘΜAg2OΩ…»ή”ΎΑ±Υ°ΓΘ
ΓΨΧα≥ω…ηœκΓΩ ‘Ιή÷–≤–ΝτΒΡΚΎ…ΪΙΧΧεΩ…Ρή «ΘΚΔΓ.AgΘΜΔΔ.Ag2OΘΜΔΘ.AgΚΆAg2OΓΘ
ΓΨ Β―ι―ι÷ΛΓΩ ΗΟ–ΓΉιΈΣ―ι÷Λ…œ ω…ηœκΘ§Ζ÷±π»Γ…ΌΝΩΚΎ…ΪΙΧΧεΖ≈»κ ‘Ιή÷–Θ§Ϋχ––ΝΥ»γœ¬ Β―ιΓΘ
Β―ι±ύΚ≈ | ≤ΌΉς | œ÷œσ |
a | Φ”»κΉψΝΩΑ±Υ°Θ§’ώΒ¥ | ΚΎ…ΪΙΧΧε≤Μ»ήΫβ |
b | Φ”»κΉψΝΩœΓœθΥαΘ§’ώΒ¥ | ΚΎ…ΪΙΧΧε»ήΫβΘ§≤Δ”–ΤχΧε≤ζ…ζ |
ΓΨ Β―ιΤάΦέΓΩ ΗυΨί…œ ω Β―ιΘ§≤ΜΡή»ΖΕ®ΙΧΧε≤ζΈο≥…Ζ÷ΒΡ Β―ι «________(Χν Β―ι±ύΚ≈)ΓΘ
ΓΨ Β―ιΫα¬έΓΩ ΗυΨί…œ ω Β―ιΫαΙϊΘ§ΗΟ–ΓΉιΒΟ≥ωAgNO3ΙΧΧε»»Ζ÷ΫβΒΡ≤ζΈο”–________ΓΘ
(1)»ή“Κ≥ ―ΣΚλ…ΪΓΓK3[Fe(CN)6]»ή“ΚΓΓ≤ζ…ζάΕ…Ϊ≥ΝΒμ
(2)ΖάΒΙΈϋ
(3)”Ο¥χΜπ–«ΒΡΡΨΧθ…λ»κΦ·ΤχΤΩΡΎΘ§ΡΨΧθΗ¥»ΦΘ§÷ΛΟςΈό…ΪΤχΧεΈΣO2
(4)bΓΓAgΓΔNO2ΓΔO2
ΓΨΫβΈωΓΩΔώ.(1)Fe3ΘΪ”ωKSCN»ή“ΚΘ§»ή“Κ±δ―ΣΚλ…ΪΓΘ”…”Ύ¬»Υ°”κFe2ΘΪΖ¥”ΠΘ§œ÷œσ≤ΜΟςœ‘Θ§”Π÷±Ϋ””ΟK3[Fe(CN)6]»ή“ΚΦλ―ιΘ§»γ”–άΕ…Ϊ≥ΝΒμ≥ωœ÷Θ§‘ρ÷ΛΟςFe2ΘΪ¥φ‘ΎΘ§Ζώ‘ρ≤Μ¥φ‘ΎΓΘ
Δρ.(2)BΉΑ÷ΟΈΣΑ≤»ΪΤΩΘ§Ω…“‘Ζά÷ΙΒΙΈϋΓΘ
(3)Φλ―ι―θΤχΒΡ≥Θ”ΟΖΫΖ® «άϊ”ΟΤδ÷ζ»Φ–‘Θ§ Ι¥χ”–Μπ–«ΒΡΡΨΧθΗ¥»ΦΓΘ
(4)a Β―ι÷–Θ§Φ”»κΑ±Υ°ΚΎ…ΪΙΧΧε≤Μ»ήΫβΘ§÷ΛΟς‘≠Έο÷ ≤ΜΚ§Ag2OΘ§Ά§ ±“≤÷ΛΟς‘≠Έο÷ ΈΣAgΘΜb Β―ι÷–Θ§÷ΜΡή÷ΛΟς‘≠ΚΎ…ΪΙΧΧεΚ§”–AgΘ§“ρAg2O“≤Ω…“‘»ήΫβ‘ΎΉψΝΩœΓœθΥα÷–Θ§Υυ“‘≤ΜΡή÷ΛΟς «ΖώΚ§”–Ag2OΓΘ
”……œ ω Β―ιΒΡΉέΚœΖ÷ΈωΩ…÷ΣΘ§AgNO3ΙΧΧε»»Ζ÷Ϋβ ±ΒΡ≤ζΈοΖ÷±πΈΣAgΓΔNO2ΚΆO2Θ§Μ·―ßΖΫ≥Χ ΫΈΣ2AgNO3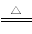 2AgΘΪ2NO2ΓϋΘΪO2Γϋ
2AgΘΪ2NO2ΓϋΘΪO2Γϋ

 –¬ΜνΝΠΉήΕ·‘± νœΒΝ–¥πΑΗ
–¬ΜνΝΠΉήΕ·‘± νœΒΝ–¥πΑΗ Νζ»ΥΆΦ ιΩλά÷ΦΌΤΎ νΦΌΉς“Β÷Θ÷ί¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗ
Νζ»ΥΆΦ ιΩλά÷ΦΌΤΎ νΦΌΉς“Β÷Θ÷ί¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗ