��Ŀ����
��Ba(OH)2��Һ����μ���ϡ���ᣬ������������⣺
(1)д����Ӧ�����ӷ���ʽ�� ��
(2)������������£����ӷ���ʽ�� (1)��ͬ���� (�����)��
A����NaHSO4��Һ����μ���Ba(OH)2��Һ����Һ������
B����NaHSO4��Һ����μ���Ba(OH)2��Һ��SO42��ǡ����ȫ����
C����NaHSO4��Һ����μ���Ba(OH)2��Һ������
(3)����������ϡ����ֱ�����������������л����Һ�ĵ�������(�õ���ǿ��I��ʾ)�ɽ��Ƶ�����ͼ�е� (�����)���߱�ʾ��

(4)����һ����⻬������С��������Ba(OH)2��Һ���룬��ͼ��ʾ������ձ��л���ע����Ba(OH)2��Һ���ܶȵ�ϡ������ǡ����ȫ��Ӧ���ڴ�ʵ������У�С�� ��

(1)д����Ӧ�����ӷ���ʽ�� ��
(2)������������£����ӷ���ʽ�� (1)��ͬ���� (�����)��
A����NaHSO4��Һ����μ���Ba(OH)2��Һ����Һ������
B����NaHSO4��Һ����μ���Ba(OH)2��Һ��SO42��ǡ����ȫ����
C����NaHSO4��Һ����μ���Ba(OH)2��Һ������
(3)����������ϡ����ֱ�����������������л����Һ�ĵ�������(�õ���ǿ��I��ʾ)�ɽ��Ƶ�����ͼ�е� (�����)���߱�ʾ��

(4)����һ����⻬������С��������Ba(OH)2��Һ���룬��ͼ��ʾ������ձ��л���ע����Ba(OH)2��Һ���ܶȵ�ϡ������ǡ����ȫ��Ӧ���ڴ�ʵ������У�С�� ��

(1)Ba2����2OH����2H����SO42��=BaSO4����2H2O��(2)A��(3)C��(4)�³�
(1)��Ba(OH)2��Һ����μ���ϡ���ᣬ��Ӧ�����ӷ���ʽΪBa2����2OH����2H����SO42��=BaSO4����2H2O��(2)��NaHSO4��Һ����μ���Ba(OH)2��Һ����Һ�����ԣ���Ӧ�����ӷ���ʽΪ2H����SO42����Ba2����2OH��=BaSO4����2H2O����NaHSO4��Һ����μ���Ba(OH)2��Һ��SO42��ǡ����ȫ���������ӷ���ʽΪ��Ba2����OH����SO42����H��=BaSO4����H2O����NaHSO4��Һ����μ���Ba(OH)2��Һ����������Ӧ�����ӷ���ʽΪ��H����SO42����Ba2����OH��=BaSO4����H2O��(3)��Ba(OH)2��Һ�л�������ϡ���ᣬ��Ba(OH)2��H2SO4ǡ����ȫ��Ӧʱ����Һ���������ӽ�Ϊ�㣬�ټӹ���ϡ���ᣬ��Һ������������ǿ��������C��ȷ��(4)Ba(OH)2��H2SO4��Ӧ�����У��淴Ӧ�Ľ��У���Һ�ܶȱ�С������С���³���

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
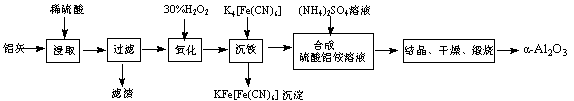
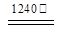 2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ����ͼ��ʾ��װ�á�
2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ����ͼ��ʾ��װ�á�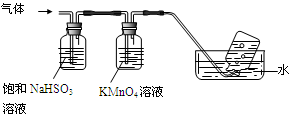
 =BaSO4��
=BaSO4��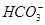 =CaCO3����
=CaCO3����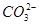 ��2H2O
��2H2O Cu(OH)2+2H+
Cu(OH)2+2H+