��Ŀ����
 ij��Ӧ����ͼ��ʾ�ķ����о����ʵ����ʣ���������A����Ҫ�ɷ��������������ǿ�����ˮ�������ش��������⣺
ij��Ӧ����ͼ��ʾ�ķ����о����ʵ����ʣ���������A����Ҫ�ɷ��������������ǿ�����ˮ�������ش��������⣺��1�������о���ʵ�飩����ҪĿ����
̽����������Ư���Ե�����
̽����������Ư���Ե�����
����2��ŨH2SO4��������
��������A�е�ˮ
��������A�е�ˮ
�����о�Ŀ��ֱ����ص�ʵ�����������ﲼ������ɫ��ʪ��IJ�����ɫ
���ﲼ������ɫ��ʪ��IJ�����ɫ
����3�����������ʵķ���������������ʵ����ƻ������¹��������¹ʱ�����
û��β������װ�ã���ɻ�����Ⱦ
û��β������װ�ã���ɻ�����Ⱦ
����4����д���˷��¹������������ķ�Ӧ����ʽ
Cl2+2NaOH=NaClO+NaCl+H2O
Cl2+2NaOH=NaClO+NaCl+H2O
����������ʵ���Ŀ����̽����������Ư���Ե������£���������������Ư���ԣ�������ˮ��Ӧ��������ʹ����ᣬ���������Ư���ԣ������ж���Ӧ����β�����������������ŷŵ������У�һ����NaOH��Һ����������
����⣺��1����ʵ��Ϊ�Ա�ʵ�飬�������ֱ��ڸ��ﲼ����ʪ�������бȽϣ����ѿ���ʵ��Ŀ����̽����������Ư���Ե��������ʴ�Ϊ��̽����������Ư���Ե�������
��2��Ũ���������ˮ�ԣ��������������������������������C�е�ˮ�����ã�������������Ư���ԣ�������ˮ��Ӧ��������ʹ����ᣬ���������Ư���ԣ��ʴ�Ϊ����������C�е�ˮ�����ﲼ������ɫ��ʪ��IJ�����ɫ��
��3�������ж���Ӧ����β�����������������ŷŵ������У��ʴ�Ϊ��û��β������װ�ã���ɻ�����Ⱦ��
��4��һ����NaOH��Һ���ն�����������䷴Ӧ�ķ���ʽΪ��Cl2+2NaOH=NaClO+NaCl+H2O���ʴ�Ϊ��Cl2+2NaOH=NaClO+NaCl+H2O��
��2��Ũ���������ˮ�ԣ��������������������������������C�е�ˮ�����ã�������������Ư���ԣ�������ˮ��Ӧ��������ʹ����ᣬ���������Ư���ԣ��ʴ�Ϊ����������C�е�ˮ�����ﲼ������ɫ��ʪ��IJ�����ɫ��
��3�������ж���Ӧ����β�����������������ŷŵ������У��ʴ�Ϊ��û��β������װ�ã���ɻ�����Ⱦ��
��4��һ����NaOH��Һ���ն�����������䷴Ӧ�ķ���ʽΪ��Cl2+2NaOH=NaClO+NaCl+H2O���ʴ�Ϊ��Cl2+2NaOH=NaClO+NaCl+H2O��
���������⿼��������Ư���Ե�̽��ʵ�飬��Ŀ�ѶȲ�����ע����������ˮ���ʵ�����

��ϰ��ϵ�д�
�����Ŀ



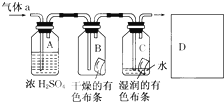 ijͬѧӦ����ͼ��ʾ�ķ����о����ʵ����ʣ���������a����Ҫ�ɷ��������������ǿ�����ˮ������
ijͬѧӦ����ͼ��ʾ�ķ����о����ʵ����ʣ���������a����Ҫ�ɷ��������������ǿ�����ˮ������