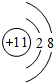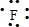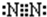��Ŀ����
A��D��ԭ��������20���ڵ�Ԫ�أ������ʻ�ṹ��Ϣ���±�| Ԫ�� | A | B | C | D |
| ���ʻ� �ṹ��Ϣ | �䵥�ʺͻ��������ɫ��Ϊ��ɫ | ���⻯���ˮ��Һ�ܸ�ʴ���� | �䵥���ڿ��������������� | �ؿ��к������Ľ���Ԫ�� |
��1��A���ӵĽṹʾ��ͼ ��Bԭ�ӵĵ���ʽ ��C�ĵ��ʵĵ���ʽΪ ��
��2����B���⻯��������������ͬ��4������ �� �� �� ��
��3��C����̬�⻯���ˮ����ĵ��뷽��ʽ�� ��
��4����Ӧ���Ӱ뾶��С�Ƚϣ�A D��
���𰸡�������A��D��ԭ��������20���ڵ�Ԫ�أ�A�ĵ��ʺͻ��������ɫ��Ϊ��ɫ����AΪNa��B���⻯���ˮ��Һ�ܸ�ʴ��������BΪF��C�ĵ����ڿ��������������࣬��CΪN��DԪ���ǵؿ��к������Ľ���Ԫ�أ���DΪAl��Ȼ���������ش�
����⣺A��D��ԭ��������20���ڵ�Ԫ�أ�A�ĵ��ʺͻ��������ɫ��Ϊ��ɫ����AΪNa��B���⻯���ˮ��Һ�ܸ�ʴ��������BΪF��C�ĵ����ڿ��������������࣬��CΪN��DԪ���ǵؿ��к������Ľ���Ԫ�أ���DΪAl��
��1��Na+�Ľṹʾ��ͼΪ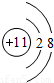 ��Fԭ�ӵ���ʽΪ
��Fԭ�ӵ���ʽΪ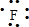 ��N2�ĵ���ʽΪ
��N2�ĵ���ʽΪ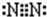 ��
��
�ʴ�Ϊ��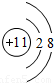 ��
��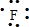 ��
��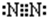 ��
��
��2��HF������������Ϊ10��������10���������У�Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+
�ʴ�Ϊ��Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+�����е�����4����ɣ�
��3��NH3��ˮ����ΪNH3?H2O������Һ�з�������NH3?H2O?NH4++OH-��
�ʴ��У�NH3?H2O?NH4++OH-��
��4��Na+��Al3+���Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶Na+��Al3+��
�ʴ�Ϊ������
���������⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ����Ŀ�ѶȲ�����ȷ�ƶ�Ԫ�ص������ǽ����Ĺؼ���Ȼ�����Ԫ�ػ�����֪ʶ���н��
����⣺A��D��ԭ��������20���ڵ�Ԫ�أ�A�ĵ��ʺͻ��������ɫ��Ϊ��ɫ����AΪNa��B���⻯���ˮ��Һ�ܸ�ʴ��������BΪF��C�ĵ����ڿ��������������࣬��CΪN��DԪ���ǵؿ��к������Ľ���Ԫ�أ���DΪAl��
��1��Na+�Ľṹʾ��ͼΪ
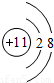 ��Fԭ�ӵ���ʽΪ
��Fԭ�ӵ���ʽΪ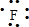 ��N2�ĵ���ʽΪ
��N2�ĵ���ʽΪ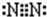 ��
���ʴ�Ϊ��
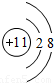 ��
��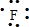 ��
��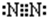 ��
�� ��2��HF������������Ϊ10��������10���������У�Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+
�ʴ�Ϊ��Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+�����е�����4����ɣ�
��3��NH3��ˮ����ΪNH3?H2O������Һ�з�������NH3?H2O?NH4++OH-��
�ʴ��У�NH3?H2O?NH4++OH-��
��4��Na+��Al3+���Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶Na+��Al3+��
�ʴ�Ϊ������
���������⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ����Ŀ�ѶȲ�����ȷ�ƶ�Ԫ�ص������ǽ����Ĺؼ���Ȼ�����Ԫ�ػ�����֪ʶ���н��

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ
A��D��ԭ��������20���ڵ�Ԫ�أ������ʻ�ṹ��Ϣ���±�
����ݱ��е���Ϣ�ش��������⣺
��1��A���ӵĵ����Ų�ʽΪ______��Bԭ�Ӻ�����ӵĹ����ʾʽΪ______��
��2����B���⻯��������������ͬ��4��������______��
��3��C�ĵ����ر��ȶ���ԭ������______��C����̬�⻯���ˮ��Һ�д��ڵ�ƽ�⣺______��
��4����ҵ����ȡ D�ĵ��ʵĻ�ѧ����ʽΪ______ 4Al+3O2��
| Ԫ�� | A | B | C | D |
| ���� �� �ṹ ��Ϣ | �䵥�ʺͻ��������ɫ��Ϊ��ɫ | ���⻯���ˮ��Һ�ܸ�ʴ���� | ʪ��ĺ�ɫʯ����ֽ������ͼ��⻯����� | ��ԭ�Ӻ�����13���˶�״̬�ĵ��� |
��1��A���ӵĵ����Ų�ʽΪ______��Bԭ�Ӻ�����ӵĹ����ʾʽΪ______��
��2����B���⻯��������������ͬ��4��������______��
��3��C�ĵ����ر��ȶ���ԭ������______��C����̬�⻯���ˮ��Һ�д��ڵ�ƽ�⣺______��
��4����ҵ����ȡ D�ĵ��ʵĻ�ѧ����ʽΪ______ 4Al+3O2��