��Ŀ����
ij�����ܱ������������淴Ӧ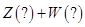
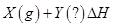 ����
����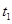 ʱ�̷�Ӧ�ﵽƽ�⣬��
ʱ�̷�Ӧ�ﵽƽ�⣬��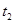 ʱ����С���������
ʱ����С��������� ʱ���ٴδﵽƽ��״̬��δ�ٸı������������й�˵������ȷ����
ʱ���ٴδﵽƽ��״̬��δ�ٸı������������й�˵������ȷ����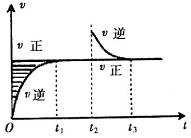
| A��Z��W�ڸ�������������һ����Ϊ��̬ |
B��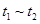 ʱ����� ʱ����� ʱ�̺���ʱ��η�Ӧ��ϵ�������ƽ��Ħ��������������� ʱ�̺���ʱ��η�Ӧ��ϵ�������ƽ��Ħ��������������� |
C�����ڸ��¶��´˷�Ӧƽ�ⳣ������ʽΪK=c��X������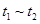 ʱ����� ʱ����� ʱ�̺��XŨ�Ȳ���� ʱ�̺��XŨ�Ȳ���� |
D�����÷�Ӧֻ��ij�¶�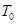 �����Է����У���÷�Ӧ��ƽ�ⳣK���¶����߶����� �����Է����У���÷�Ӧ��ƽ�ⳣK���¶����߶����� |
D
�������������A������ͼ���֪������Ӧ���ʲ��淴Ӧʱ���ѹǿ�ĸı���ı䣬��Z��W���Dz������壬��A����B�����ͼ���֪��ֻ��X�����壬���Է�Ӧ�����������Ħ������ʼ�ղ��䣬��B����C�����ڻ�ѧƽ�ⳣ��ֻ���¶��йأ����¶���ƽ�ⳣ���ı���ʽK=c��X���Ƕ�ֵ����t1��t2ʱ�����t3ʱ�̺��c��X����ȣ���C����D�����ڸ÷�Ӧ���¶�ΪT0����ʱ�����Է����У����ݡ�H-T��S��0���ó��÷�Ӧ�����ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ������D��ȷ��
���㣺��ѧƽ���Ӱ������

 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д���һ�������£�����A2(g)��3B2(g) 2AB3(g)��Ӧ��˵�����»�ѧ��Ӧ���ʵı�ʾ�У���ѧ��Ӧ����������
2AB3(g)��Ӧ��˵�����»�ѧ��Ӧ���ʵı�ʾ�У���ѧ��Ӧ����������
| A��v(A2)��0.8 mol��L��1��s��1 | B��v(A2)��30 mol��L��1��min��1 |
| C��v(AB3)��1.0 mol��L��1��s��1 | D��v(B2)��1.2 mol��L��1��s��1 |
���ܱ������з�����Ӧ��4NH3��g����5O2��g�� 4NO��g����6H2O��g�� ��H��0�����д�ʩ��ʹƽ�������ƶ�����
4NO��g����6H2O��g�� ��H��0�����д�ʩ��ʹƽ�������ƶ�����
| A������ѹǿ | B�������¶� | C��������� | D������O2��Ũ�� |
���ݻ�һ�����ܱ������У����п��淴ӦN2(g)+3H2(g)  2NH3(g) ��H��0����ͼ����ʾ�ķ�Ӧ���ߣ�T��ʾ�¶ȣ�P��ʾѹǿ��C%��ʾNH3�����������������˵���в���ȷ����
2NH3(g) ��H��0����ͼ����ʾ�ķ�Ӧ���ߣ�T��ʾ�¶ȣ�P��ʾѹǿ��C%��ʾNH3�����������������˵���в���ȷ����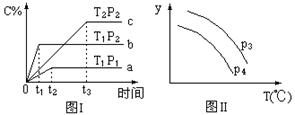
| A��T2��T1 |
| B��P2��P1 |
| C����P3��P4��y���ʾN2��ת���� |
| D����P3��P4��y���ʾNH3��������� |
���ݻ�һ�����ܱ������з������淴Ӧ��A��g����2B��g�� 2C��g�� ��H��0�������������仯��ֻ���¶ȱ仯ʱ��ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵���У���ȷ����
2C��g�� ��H��0�������������仯��ֻ���¶ȱ仯ʱ��ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵���У���ȷ����
| A����P1��P2���������ʾA���������� |
| B����P1��P2���������ʾC���������� |
| C����P1��P2���������ʾ��������ƽ��Ħ������ |
| D����P1��P2���������ʾA��ת���� |
ij�¶��£�H2(g)��CO2(g)? ?H2O(g)��CO(g)��ƽ�ⳣ��K��9/4�����¶����ڼס��ҡ������������ܱ������У�Ͷ��H2(g)��CO2(g)������ʼŨ�������ʾ�������жϲ���ȷ����
?H2O(g)��CO(g)��ƽ�ⳣ��K��9/4�����¶����ڼס��ҡ������������ܱ������У�Ͷ��H2(g)��CO2(g)������ʼŨ�������ʾ�������жϲ���ȷ����
| ��ʼŨ�� | �� | �� | �� |
| c(H2)/mol��L��1 | 0.010 | 0.020 | 0.020 |
| c(CO2)/mol��L��1 | 0.010 | 0.010 | 0.020 |
A��ƽ��ʱ������CO2��ת���ʴ���60%
B��ƽ��ʱ�����кͱ���H2��ת���ʾ���60%
C��ƽ��ʱ������c(CO2)�Ǽ��е�2������0.012 mol��L��1
D����Ӧ��ʼʱ�����еķ�Ӧ������죬���еķ�Ӧ��������
�ظ������Һ�д�������ƽ�⣺Cr2O72-(�Ⱥ�ɫ)+H2O  2H++2CrO42-(��ɫ)����K2Cr2O7��Һ�м������������Լ�����Һ��ɫ��ӻ�ɫ��Ϊ�Ⱥ�ɫ
2H++2CrO42-(��ɫ)����K2Cr2O7��Һ�м������������Լ�����Һ��ɫ��ӻ�ɫ��Ϊ�Ⱥ�ɫ
| A��NaHSO4 | B��NaHCO3 | C��Na2CO3 | D��CH3COONa |
 xC(g) ��H��0�����������c(A)��ʱ��t�ı仯��ͼ��ʾ��
xC(g) ��H��0�����������c(A)��ʱ��t�ı仯��ͼ��ʾ��
 2C(g)+2D(g)���ڲ�ͬ����²�÷�Ӧ���ʣ����з�Ӧ���������ǣ� ��
2C(g)+2D(g)���ڲ�ͬ����²�÷�Ӧ���ʣ����з�Ӧ���������ǣ� ��