��Ŀ����
��֪2SO2 (g) + O2 (g)  2SO3 (g)����H����197 kJ��mol-1����ͬ�¡�ͬ����������ܱ������зֱ�������壺(��) 2 mol SO2��1 mol O2��(��) 1 mol SO2��0.5 mol O2��(��) 2 mol SO3�����¡������·�Ӧ��ƽ��ʱ�����й�ϵһ����ȷ���ǣ� ��
2SO3 (g)����H����197 kJ��mol-1����ͬ�¡�ͬ����������ܱ������зֱ�������壺(��) 2 mol SO2��1 mol O2��(��) 1 mol SO2��0.5 mol O2��(��) 2 mol SO3�����¡������·�Ӧ��ƽ��ʱ�����й�ϵһ����ȷ���ǣ� ��
| A��������ѹǿP��P����P�� > 2P�� |
| B��SO3������m��m����m�� > 2m�� |
| C��c(SO2)��c(O2)֮��k��k����k�� > k�� |
| D����Ӧ�ų���������������ֵQ��Q����G�� > 2Q�� |
B
����������������������֪�ͱ������ǵ�Ч�ģ��������൱����������Ϊԭ����һ�룬�����������ڼ�Сѹǿ�Ĺ�����ƽ�������ƶ���ѹǿ��ԭ����һ�������p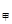 =p
=p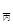 <2p
<2p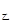 ��SO3��������ԭ����һ��С������m
��SO3��������ԭ����һ��С������m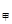 =m
=m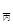 >2m
>2m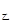 ��c(SO
��c(SO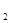 )��c(O
)��c(O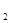 )֮��k���䣬����k
)֮��k���䣬����k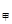 =k
=k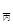 =k
=k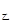 ���ų���������ԭ����һ��С������Q
���ų���������ԭ����һ��С������Q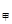 >2Q
>2Q ��Q��+Q��=197��������ȷ����B����ѡB��
��Q��+Q��=197��������ȷ����B����ѡB��
���㣺��ѧ��Чƽ��
����������Ĺؼ��Ǽ��������ıȽϣ��������൱�ڼ������������Ϊԭ����һ�룬Ȼ���ٽ��бȽϡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���ǣ� ��
A����֪2SO2(g)+O2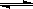 2SO3(g) Ϊ���ȷ�Ӧ����SO2������һ������SO3������ 2SO3(g) Ϊ���ȷ�Ӧ����SO2������һ������SO3������ |
| B����֪C(ʯī,s) ===C�����ʯ,s�� ��H��0������ʯ��ʯī�ȶ� |
| C��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.4 kJ/mol����20 g NaOH����Һ������ϡ������ȫ��Ӧ���ų�������Ϊ28.7 kJ |
| D����֪2C(s) +2O2 (g) ===2CO2(g) ��H1 |
 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺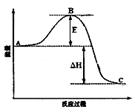
 2SO3(g)(����Ӧ����)������500��ʹ����������£��÷�Ӧ���ݻ��̶����ܱ������н��У������й�˵����ȷ����
2SO3(g)(����Ӧ����)������500��ʹ����������£��÷�Ӧ���ݻ��̶����ܱ������н��У������й�˵����ȷ����