��Ŀ����
L-��Ϳ���������ɭ�ۺ�֢�����ƣ�����ҩ��������ǻ��ڻ��2000��ŵ��������ѧ��ҽѧ���ͻ��2001��ŵ������ѧ�����о��ɹ������3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����֪��ͬ������L-��������ڱ�״���µ����Ϊ0.448L���Իش���1��L-��͵Ļ�ѧʽ�� ��
��2����L-��ͷ����뱽��������Ӿ������ƽṹ��������䱽����������ȡ������������������ͬȡ����������λ������֪�����ʣ��ټȾ������ԣ��־��м��ԣ��������Ȼ�����Һ����ɫ����1molL-��Ϳ���3mol NaOHǡ�÷�Ӧ����L-��͵Ľṹ��ʽ�ǣ� ��
��3��ij�л���A����ѧʽΪC3H7O2N����L-��͵�����������֮����A�ж���ͬ���칹�壬��д�������к���-COO-�ṹ��A��ͬ���칹��Ľṹ��ʽ��ֻд��3�֣� �� �� ��
���𰸡�����������Ԫ�������������йز����������������㣺L-��������ڱ�״���µ����Ϊ0.448L�������ʵ���Ϊ =0.02mol��L-�����Է�������Ϊ
=0.02mol��L-�����Է�������Ϊ =197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��=
=197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��= =0.02mol��n��H��=2×
=0.02mol��n��H��=2×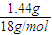 +3×0.02mol=0.22mol��
+3×0.02mol=0.22mol��
���������غ��֪��m��O��=3.94g-m��C��-m��H��-m��N��=3.94g-12g/mol×0.18mol-1g/mol×0.22mol-14g/mol×0.02mol=1.28g������n��O��= =0.08mol�����ԣ�1 mol L-��ͺ���n��C��=9 mol��n��H��=11 mol��n��N��=l mol��n��O��=4 mol������L-��͵ķ���ʽΪC9H11O4N��
=0.08mol�����ԣ�1 mol L-��ͺ���n��C��=9 mol��n��H��=11 mol��n��N��=l mol��n��O��=4 mol������L-��͵ķ���ʽΪC9H11O4N��
��2���������ʷ��������뱽������������ƣ��Ⱦ����������м��ԣ����ǰ���������Ȼ�����Һ����ɫ��˵�����з��ǻ���1 mol L-�����3 mol NaOHǡ����ȫ��Ӧ��˵�����еķ��ǻ����Ȼ���3 mol����������������ȡ����������������ͬȡ����������λ����Ϸ���ʽ��֪�������������ǻ���һ���Ȼ���
��3��ij�л���A����ѧʽΪC3H7O2N����L-��͵�����������֮������ṹ��Ӧ���а������Ȼ����������Դ���дͬ���칹��Ľṹ��ʽ��
����⣺��1��L-��������ڱ�״���µ����Ϊ0.448L�������ʵ���Ϊ =0.02mol��L-�����Է�������Ϊ
=0.02mol��L-�����Է�������Ϊ =197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��=
=197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��= =0.02mol��n��H��=2×
=0.02mol��n��H��=2× +3×0.02mol=0.22mol��
+3×0.02mol=0.22mol��
���������غ��֪��m��O��=3.94g-m��C��-m��H��-m��N��=3.94g-12g/mol×0.18mol-1g/mol×0.22mol-14g/mol×0.02mol=1.28g������n��O��= =0.08mol��
=0.08mol��
���ԣ�1 mol L-��ͺ���n��C��=9 mol��n��H��=11 mol��n��N��=l mol��n��O��=4 mol��
��L-��͵ķ���ʽΪC9H11O4N��
�ʴ�Ϊ��C9HllO4N��
��2���������ʷ��������뱽������������ƣ��Ⱦ����������м��ԣ����ǰ���������Ȼ�����Һ����ɫ��˵�����з��ǻ���1 mol L-�����3 mol NaOHǡ����ȫ��Ӧ��˵�����еķ��ǻ����Ȼ���3 mol����������������ȡ����������������ͬȡ����������λ����Ϸ���ʽ��֪�������������ǻ���һ���Ȼ�����ṹ��ʽΪ ��
��
�ʴ�Ϊ��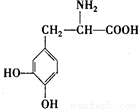 ��
��
��3���л���A����ѧʽΪC3H7O2N����L-��͵�����������֮������ṹ��Ӧ���а������Ȼ���������
����ܵĽṹΪ��CH3CH��NH2��COOH��H2NCH2CH2COOH��HCOOCH��NH2��CH3��HCOOCH2CH2NH2��H2NCH2COOCH3��CH3COOCH2NH2�ȣ�
�ʴ�Ϊ��CH3CH��NH2��COOH��H2NCH2CH2COOH��HCOOCH��NH2��CH3��HCOOCH2CH2NH2��H2NCH2COOCH3��CH3COOCH2NH2����ѡ���֣���
���������⿼���л�����ӵ�ȷ������Ŀ�Ѷ��еȣ�����ע����л���ȼ�������������������ж�����Ԫ�ص����ʵ�����ϵ�����������غ�ȷ���Ƿ�����Ԫ�أ���������ȷ���л�����ܵĽṹ��
 =0.02mol��L-�����Է�������Ϊ
=0.02mol��L-�����Է�������Ϊ =197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��=
=197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��= =0.02mol��n��H��=2×
=0.02mol��n��H��=2×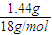 +3×0.02mol=0.22mol��
+3×0.02mol=0.22mol�����������غ��֪��m��O��=3.94g-m��C��-m��H��-m��N��=3.94g-12g/mol×0.18mol-1g/mol×0.22mol-14g/mol×0.02mol=1.28g������n��O��=
 =0.08mol�����ԣ�1 mol L-��ͺ���n��C��=9 mol��n��H��=11 mol��n��N��=l mol��n��O��=4 mol������L-��͵ķ���ʽΪC9H11O4N��
=0.08mol�����ԣ�1 mol L-��ͺ���n��C��=9 mol��n��H��=11 mol��n��N��=l mol��n��O��=4 mol������L-��͵ķ���ʽΪC9H11O4N����2���������ʷ��������뱽������������ƣ��Ⱦ����������м��ԣ����ǰ���������Ȼ�����Һ����ɫ��˵�����з��ǻ���1 mol L-�����3 mol NaOHǡ����ȫ��Ӧ��˵�����еķ��ǻ����Ȼ���3 mol����������������ȡ����������������ͬȡ����������λ����Ϸ���ʽ��֪�������������ǻ���һ���Ȼ���
��3��ij�л���A����ѧʽΪC3H7O2N����L-��͵�����������֮������ṹ��Ӧ���а������Ȼ����������Դ���дͬ���칹��Ľṹ��ʽ��
����⣺��1��L-��������ڱ�״���µ����Ϊ0.448L�������ʵ���Ϊ
 =0.02mol��L-�����Է�������Ϊ
=0.02mol��L-�����Է�������Ϊ =197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��=
=197����3.94g L-����ڿ�������ȫȼ�տ�����0.18mol CO2��0.448LNH3����״������1.44g H2O����n��NH3��= =0.02mol��n��H��=2×
=0.02mol��n��H��=2× +3×0.02mol=0.22mol��
+3×0.02mol=0.22mol�����������غ��֪��m��O��=3.94g-m��C��-m��H��-m��N��=3.94g-12g/mol×0.18mol-1g/mol×0.22mol-14g/mol×0.02mol=1.28g������n��O��=
 =0.08mol��
=0.08mol�����ԣ�1 mol L-��ͺ���n��C��=9 mol��n��H��=11 mol��n��N��=l mol��n��O��=4 mol��
��L-��͵ķ���ʽΪC9H11O4N��
�ʴ�Ϊ��C9HllO4N��
��2���������ʷ��������뱽������������ƣ��Ⱦ����������м��ԣ����ǰ���������Ȼ�����Һ����ɫ��˵�����з��ǻ���1 mol L-�����3 mol NaOHǡ����ȫ��Ӧ��˵�����еķ��ǻ����Ȼ���3 mol����������������ȡ����������������ͬȡ����������λ����Ϸ���ʽ��֪�������������ǻ���һ���Ȼ�����ṹ��ʽΪ
 ��
���ʴ�Ϊ��
 ��
����3���л���A����ѧʽΪC3H7O2N����L-��͵�����������֮������ṹ��Ӧ���а������Ȼ���������
����ܵĽṹΪ��CH3CH��NH2��COOH��H2NCH2CH2COOH��HCOOCH��NH2��CH3��HCOOCH2CH2NH2��H2NCH2COOCH3��CH3COOCH2NH2�ȣ�
�ʴ�Ϊ��CH3CH��NH2��COOH��H2NCH2CH2COOH��HCOOCH��NH2��CH3��HCOOCH2CH2NH2��H2NCH2COOCH3��CH3COOCH2NH2����ѡ���֣���
���������⿼���л�����ӵ�ȷ������Ŀ�Ѷ��еȣ�����ע����л���ȼ�������������������ж�����Ԫ�ص����ʵ�����ϵ�����������غ�ȷ���Ƿ�����Ԫ�أ���������ȷ���л�����ܵĽṹ��

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ

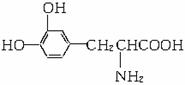
 ����ҩ��������ǻ��ڻ��2000��ŵ��������ѧ��ҽѧ���ͻ��2001��ŵ������ѧ�����о��ɹ������й���L-�������Ե�������ȷ���ǣ�������
����ҩ��������ǻ��ڻ��2000��ŵ��������ѧ��ҽѧ���ͻ��2001��ŵ������ѧ�����о��ɹ������й���L-�������Ե�������ȷ���ǣ������� L-�����һ���л��������������ɭ�ۺ�֢�����ƣ���ṹ��ʽ��ͼ������ҩ��������ǻ��ڻ��2000��ŵ��������ѧ��ҽѧ���ͻ��2001��ŵ������ѧ�����о��ɹ������й���L-��͵���������ȷ���ǣ�������
L-�����һ���л��������������ɭ�ۺ�֢�����ƣ���ṹ��ʽ��ͼ������ҩ��������ǻ��ڻ��2000��ŵ��������ѧ��ҽѧ���ͻ��2001��ŵ������ѧ�����о��ɹ������й���L-��͵���������ȷ���ǣ�������