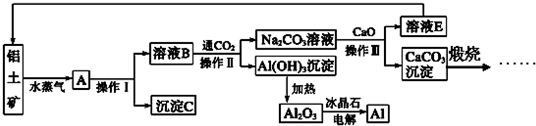
��Ŀ����
��ش��������⣮
��1��ˮ�д���ƽ�⣺H2O?H++OH-��H��0����ʹƽ�����ƣ�����Һ�����ԣ���ѡ�ķ�����______����ѡ����ţ�
a����ˮ�м���NaHSO4���� b����ˮ�м�Na2CO3����
c��������100��d����ˮ�м��� ��NH4��2SO4����
��2��A��B��C��D 4��������ˮ��ǿ�������ˮ�пɵ�������������ӣ��������Ӳ��ظ�����
�ٲ������κ�ʵ�鼴���жϣ���4�ֻ�������2����______��______���ѧʽ������2�ֻ��������Һ���ʱ��Ӧ�����ӷ���ʽΪ______��
��Ϊȷ��A��B��C��D�ijɷ֣�ij̽����ѧϰС���������ʵ�飬�����ʵ����ۣ�
��1��ˮ�д���ƽ�⣺H2O?H++OH-��H��0����ʹƽ�����ƣ�����Һ�����ԣ���ѡ�ķ�����______����ѡ����ţ�
a����ˮ�м���NaHSO4���� b����ˮ�м�Na2CO3����
c��������100��d����ˮ�м��� ��NH4��2SO4����
��2��A��B��C��D 4��������ˮ��ǿ�������ˮ�пɵ�������������ӣ��������Ӳ��ظ�����
| ������ | Ba2+��H+��Na+��Mg2+ |
| ������ | Cl-��SO42-��CO32-��OH- |
��Ϊȷ��A��B��C��D�ijɷ֣�ij̽����ѧϰС���������ʵ�飬�����ʵ����ۣ�
| ʵ����� | ʵ������ | ʵ����� |
| 1 | ��pH��ֽ���A��C����Һ�ʼ��ԣ�B��D����Һ������ | |
| 2 | A��Һ��B��Һ���ʱ������ɫ���ݲ��� | ������AΪ______���ѧʽ��B��Һ�п϶���������______�������ӷ��ţ��� |
| 3 | C��D����Һ���ʱ���а�ɫ�������ɣ����ˣ�������еμӹ������ᣬ���������ܽ� | ������BΪ______��������DΪ______���ѧʽ���� |
��1��ˮ�д���ƽ�⣺H2O?H++OH-��H��0����ʹƽ�����ƣ�����Һ�����ԣ�˵����������ˮ������������������������ʣ���Һ�����ԣ�
a����ˮ�м���NaHSO4���壬����ˮ�ĵ��룬ƽ��������У���a�����ϣ�
b����ˮ�м�Na2CO3���壬̼�������ˮ��ٽ�ˮ�ĵ��룬��Һ�ʼ��ԣ���b�����ϣ�
c��������100�棬�ٽ�ˮ�ĵ��룬��Һ�����ԣ���c�����ϣ�
d����ˮ�м��� ��NH4��2SO4���壬笠�����ˮ��ٽ�ˮ�ĵ��룬��Һ�����ԣ���d���ϣ�
��ѡd��
��2����A��B��C��D 4��������ˮ��ǿ�������ˮ�пɵ�������������ӣ��������ӹ�������жϣ���������̼�������ֻ�ܺ��������������̼���ƣ�����������ֻ�ܺͱ������������������������2�ֻ��������Һ���ʱ��Ӧ�����ӷ���ʽ��Ba2++CO32-=BaCO3�����ʴ�Ϊ��Na2CO3��Ba��OH��2 ��Ba2++CO32-=BaCO3��
����pH��ֽ���A��C����Һ�ʼ��ԣ�����ΪNa2CO3��Ba��OH��2 ��B��D����Һ������ΪMgCl2��H2SO4��MgSO4��HCl��
A��Һ��B��Һ���ʱ������ɫ���ݲ�����֤��AΪNa2CO3��BΪ�Ậ��H+��CΪBa��OH��2 ��
C��D����Һ���ʱ���а�ɫ�������ɣ����ˣ�������еμӹ������ᣬ���������ܽ⣬�ܽ�ij�����������þ���������ܽ�������ᱵ���ж�D��ΪMgSO4��BΪHCl��
�ʴ�Ϊ��Na2CO3 ��H+��HCl��MgSO4��
a����ˮ�м���NaHSO4���壬����ˮ�ĵ��룬ƽ��������У���a�����ϣ�
b����ˮ�м�Na2CO3���壬̼�������ˮ��ٽ�ˮ�ĵ��룬��Һ�ʼ��ԣ���b�����ϣ�
c��������100�棬�ٽ�ˮ�ĵ��룬��Һ�����ԣ���c�����ϣ�
d����ˮ�м��� ��NH4��2SO4���壬笠�����ˮ��ٽ�ˮ�ĵ��룬��Һ�����ԣ���d���ϣ�
��ѡd��
��2����A��B��C��D 4��������ˮ��ǿ�������ˮ�пɵ�������������ӣ��������ӹ�������жϣ���������̼�������ֻ�ܺ��������������̼���ƣ�����������ֻ�ܺͱ������������������������2�ֻ��������Һ���ʱ��Ӧ�����ӷ���ʽ��Ba2++CO32-=BaCO3�����ʴ�Ϊ��Na2CO3��Ba��OH��2 ��Ba2++CO32-=BaCO3��
����pH��ֽ���A��C����Һ�ʼ��ԣ�����ΪNa2CO3��Ba��OH��2 ��B��D����Һ������ΪMgCl2��H2SO4��MgSO4��HCl��
A��Һ��B��Һ���ʱ������ɫ���ݲ�����֤��AΪNa2CO3��BΪ�Ậ��H+��CΪBa��OH��2 ��
C��D����Һ���ʱ���а�ɫ�������ɣ����ˣ�������еμӹ������ᣬ���������ܽ⣬�ܽ�ij�����������þ���������ܽ�������ᱵ���ж�D��ΪMgSO4��BΪHCl��
�ʴ�Ϊ��Na2CO3 ��H+��HCl��MgSO4��

��ϰ��ϵ�д�
�����Ŀ
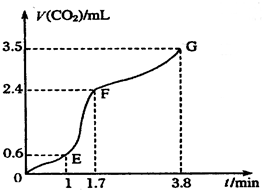 ��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺
��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺